
ABFENTIQ 600 MICROGRAMOS COMPRIMIDOS PARA CHUPAR EFG

Cómo usar ABFENTIQ 600 MICROGRAMOS COMPRIMIDOS PARA CHUPAR EFG
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
PROSPECTO : INFORMACIÓN PARA EL USUARIO
Abfentiq 200 microgramos comprimidos para chupar EFG
Abfentiq 400 microgramos comprimidos para chupar EFG
Abfentiq 600 microgramos comprimidos para chupar EFG
Abfentiq 800 microgramos comprimidos para chupar EFG
Abfentiq 1200 microgramos comprimidos para chupar EFG
Abfentiq 1600 microgramos comprimidos para chupar EFG
Fentanilo
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o farmacéutico.
- Este medicamento se le ha recetado a usted y no debe dárselo a otras personas, aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico o farmacéutico incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto:
- Qué es Abfentiq y para qué se utiliza
- Qué necesita saber antes de empezar a usar Abfentiq
- Cómo usar Abfentiq
- Posibles efectos adversos
5 Conservación de Abfentiq
- Contenido del envase e información adicional.
1. Qué es Abfentiq y para qué se utiliza
Abfentiq contiene como principio activo, fentanilo, un potente analgésico que pertenece al grupo de los opiáceos. Abfentiq se presenta en comprimidos para chupar con aplicador bucal integrado.
- Abfentiq está indicado en el tratamiento del dolor irruptivo en pacientes adultos con cáncer, y que ya están tomando otros medicamentos opiáceos para el dolor persistente (presente todo el tiempo) que les provoca el cáncer. El dolor irruptivo es un dolor súbito adicional que aparece sobre un dolor de base, y que se manifiesta a pesar de haber tomado los medicamentos analgésicos opiáceos usuales.
- No use Abfentiq si no ha estado tomando regularmente todos los días, un medicamento opioide recetado para el dolor persistente (presente todo el tiempo), durante al menos una semana, puesto que si no ha estado tomando esos medicamentos, el uso de Abfentiq puede aumentar las probabilidades de que su respiración se vuelva más lenta y/o poco profunda, e incluso de que llegue a pararse.
- No use Abfentiq para tratar el dolor causado por heridas, cirugía, dolor de cabeza o migrañas.
2. Qué necesita saber antes de empezar a usar Abfentiq
No use Abfentiq
- si no está usando regularmente un medicamento opioide prescrito por su médico (p. ej., codeína, fentanilo, hidromorfona, morfina, oxicodona, meperidina), todos los días a la misma hora, al menos durante una semana, para controlar el dolor persistente. Si no ha estado usando esos medicamentos, no use Abfentiq dado que su uso puede aumentar el riesgo de que su respiración se vuelva más lenta y/o superficial, e incluso de que llegue a pararse
- Si es alérgico (hipersensible) al fentanilo, o a cualquiera de los demás componentes de Abfentiq (incluidos en la Sección 6).
- Si está actualmente tomando medicamentos inhibidores de monoamina-oxidasa (IMAO) para el tratamiento de la depresión grave (o lo ha hecho hace menos de 2 semanas).
- Si padece problemas respiratorios graves o una afección pulmonar obstructiva grave.
- Si padece dolor de corta duración distinto al dolor irruptivo
No use Abfentiq si se encuentra en cualquiera de los casos anteriores. Si no está seguro, consulte a su médico o farmacéutico antes de utilizar Abfentiq.
Advertencias y precauciones:
Durante el tratamiento con Abfentiq siga utilizando el medicamento opioide analgésico que toma para su dolor persistente (presente todo el tiempo) asociado al cáncer.
Si usted reúne alguna de las siguientes condiciones, consulte con su médico o farmacéutico antes de usar Abfentiq:
- Si el otro medicamento opioide que toma para su dolor persistente (presente todo el tiempo) asociado al cáncer aún no se ha estabilizado.
- Si usted padece alguna enfermedad que le afecte la respiración (como asma, silbidos respiratorios, o falta de aliento).
- Si tiene alguna lesión en la cabeza o ha sufrido alguna pérdida de conciencia.
- Si tiene problemas de corazón, especialmente frecuencia cardiaca baja.
- Si tiene problemas del hígado o riñones, ya que éstos afectan el modo en que el organismo elimina el medicamento.
- Si tiene la presión sanguínea baja debido a un bajo volumen de líquido en el sistema circulatorio.
- Si es diabético.
- Si tiene más de 65 años, ya que puede ser preciso disminuir la dosis. Cualquier incremento en la dosis deberá ser cuidadosamente supervisado por su médico.
- Si toma antidepresivos o antipsicóticos; consulte la sección “Uso de otros medicamentos”.
- Si toma analgésicos para el dolor neuropático (gabapentina y pregabalina).
Es posible que su médico necesite vigilarlo de forma más estrecha:
- Si usted o algún familiar suyo han presentado alguna vez abuso o dependencia de alcohol, medicamentos de venta con receta o drogas (“adicción”).
- Si es fumador.
- Si ha tenido alguna vez problemas relacionados con el estado de ánimo (depresión, ansiedad o trastorno de la personalidad) o ha sido tratado por un psiquiatra por otras enfermedades mentales.
Consulte a su médico si DURANTE el uso de Abfentiq:
- Siente dolor o mayor sensibilidad al dolor (hiperalgesia) que no responde a una dosis más alta del medicamento tal como se lo recetó el médico.
- Presenta una combinación de los siguientes síntomas: náuseas, vómitos, anorexia, fatiga, debilidad, mareo y presión arterial baja. Juntos, estos síntomas pueden ser una indicación de una afección potencialmente mortal denominada insuficiencia suprarrenal, en la que las glándulas suprarrenales no producen suficientes hormonas.
- Alguna vez ha presentado insuficiencia suprarrenal o falta de hormonas sexuales (deficiencia de andrógenos) con el uso de opioides.
- Si presenta trastornos respiratorios relacionados con el sueño: Abfentiq puede causar trastornos respiratorios relacionados con el sueño tales como apnea del sueño (pausas respiratorias durante el sueño) e hipoxemia relacionada con el sueño (nivel bajo de oxígeno en la sangre). Los síntomas pueden incluir pausas respiratorias durante el sueño, despertar nocturno debido a la dificultad para respirar, dificultad para mantener el sueño o somnolencia excesiva durante el día. Si usted u otra persona observan estos síntomas, póngase en contacto con su médico. Es posible que su médico considere la posibilidad de reducir la dosis.
- El uso repetido de Abfentiq puede dar lugar a dependencia y abuso que pueden causar una sobredosis potencialmente mortal. Si está preocupado por la posibilidad de adquirir dependencia de Abfentiq, es importante que consulte a su médico.
Niños y adolescentes
Abfentiq no está recomendado para niños de edad inferior a los 16 años.
Uso en deportistas
Este medicamento contiene fentanilo que puede producir un resultado positivo en las pruebas de control de dopaje.
Uso de Abfentiq con otros medicamentos
Informe a su médico o farmacéutico si está utilizando o ha utilizado recientemente alguno de los siguientes medicamentos:
- Otros tratamientos a base de fentanilo que le hubieran prescrito anteriormente para el dolor irruptivo. Si aún conserva estos productos de fentanilo en casa, contacte con el farmacéutico quien le indicará cómo desprenderse de ellos.
Informe a su médico o farmacéutico si está utilizando o ha utilizado recientemente otros medicamentos, incluso los adquiridos sin receta y las plantas medicinales. En particular, informe a su médico o farmacéutico si está utilizando alguno de los siguientes medicamentos:
- Algún medicamento que pueda producirle sueño, como píldoras para dormir, medicamentos para tratar la ansiedad, algunos medicamentos para tratar la alergia (antihistamínicos) o tranquilizantes.
- Ciertos relajantes musculares (como baclofen o diazepam).
- Algún medicamento que pueda afectar al modo en que el organismo elimina Abfentiq, tales como ritonavir u otros medicamentos que ayudan a controlar la infección por el virus VIH (SIDA) u otros medicamentos denominados “inhibidores de la CYP3A4” como el ketoconazol, itraconazol o fluconazol (utilizados para las infecciones por hongos) y troleandomicina, claritromicina o eritromicina (medicamentos para las infecciones bacterianas) y los llamados “inductores de CYP3A4” como rifampicina o rifabutina 8medicamentos para las infecciones bacterianas), carbamazepina, fenobarbital o fenitoína (medicamentos utilizados para tratar las convulsiones/ataques)
- Cualquier medicamento que pueda reducir o revertir el efecto de Abfentiq (como naloxona, pentazocina, buprenorfina). Pueden causar la aparición de síndrome de abstinencia.
- Si debe someterse a cirugía que requiera anestesia general.
- El riesgo de efectos adversos aumenta si está tomando medicamentos tales como ciertos antidepresivos o antipsicóticos. Abfentiq puede interactuar con estos medicamentos y usted puede presentar cambios en el estado mental (p. ej., agitación, alucinaciones, coma) y otros efectos como temperatura corporal mayor de 38°C, aumento de la frecuencia cardiaca, presión arterial inestable y exageración de los reflejos, rigidez muscular, falta de coordinación y/o síntomas gastrointestinales (p. ej., náuseas, vómitos, diarrea). Su médico le dirá si Abfentiq es adecuado para usted.
Uso de Abfentiq con sedantes:
El uso concomitante de Abfentiq y medicamentos sedantes, tales como las benzodiacepinas o medicamentos relacionados, aumenta el riesgo de somnolencia, dificultad al respirar (depresión respiratoria), coma y puede poner en riesgo la vida. Por lo tanto, el uso concomitante únicamente debe considerarse cuando no hay otras alternativas posibles de tratamiento.
Sin embargo, si su médico le ha prescrito Abfentiq y medicamentos sedantes simultáneamente, la dosis y duración del tratamiento deberán estar limitadas por su médico.
Informe a su médico de todos los medicamentos sedantes que esté tomando y siga estrictamente la dosis recomendada por su médico. Puede ser útil informar a su familia o amigos de los signos y síntomas mencionados anteriormente. Hable con su médico si experimenta alguno de estos síntomas.
Uso de Abfentiq con alimentos, bebidas y alcohol
- Abfentiq puede usarse antes o después de las comidas. Sin embargo, no lo utilice durante una comida.
- Puede beber un poco de agua antes de usar Abfentiq para humedecerse la boca. Sin embargo, no debe beber ni comer nada mientras usa Abfentiq.
- No tome zumo de pomelo mientras use Abfentiq ya que puede tener efecto sobre el modo en que el organismo elimina Abfentiq.
- No tome bebidas alcohólicas mientras esté en tratamiento con Abfentiq ya que puede aumentar las probabilidades de sufrir graves efectos adversos.
Embarazo, lactancia y fertilidad
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su
No use Abfentiq durante el parto porque puede provocar dificultades respiratorias en el recién nacido. Hay también un riesgo de que el recién nacido sufra síndrome de abstinencia si durante el embarazo ha utilizado Abfentiq durante largos períodos de tiempo.
El fentanilo puede pasar a la leche materna y causar efectos adversos en el lactante. No use Abfentiq si está dando el pecho a su hijo. No debe iniciar la lactancia antes de transcurridas 48 horas desde la última dosis de Abfentiq.
Consulte a su médico o farmacéutico antes de utilizar cualquier medicamento, si está embarazada o en período de lactancia.
Conducción y uso de máquinas
Este medicamento puede afectar su habilidad para conducir o manejar determinadas herramientas o maquinaria. Consulte con su médico sobre la seguridad para usted de conducir o manejar determinadas herramientas o maquinaria en las horas siguientes al uso de Abfentiq.
No conduzca o maneje determinadas herramientas o maquinaria si: se siente adormecido o mareado; tiene visión borrosa o doble visión; tiene dificultad en concentrarse. Es importante que conozca como le afecta Abfentiq antes de conducir o manejar determinadas herramientas o maquinaria.
Abfentiq contiene glucosa y sodio.
- Este medicamento contiene glucosa. Si su médico le ha indicado que padece una intolerancia a ciertos azúcares, consulte con él antes de tomar este medicamento. Puede producir caries.
Este medicamento contiene menos de 23 mg de sodio (1mmol) por comprimido para chupar; esto es, esencialmente “exento de sodio”.
3. Cómo usar Abfentiq
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico o farmacéutico. En caso de duda, pregunte a su médico o farmacéutico.
Cuando empiece a utilizar Abfentiq por primera vez, su médico colaborará con usted para encontrar la dosis de Abfentiq que le alivie el dolor irruptivo. Es muy importante que utilice Abfentiq exactamente como se lo ha indicado su médico.
- No cambie por su cuenta las dosis de Abfentiq ni de otros analgésicos. Cualquier cambio en la dosificación tiene que ser prescrito y vigilado por su médico.
- Si tiene dudas sobre la dosis correcta o si tiene preguntas sobre el uso de Abfentiq, consúltelo a su médico.
Cómo penetra el medicamento en el organismo
Cuando usted se pone Abfentiq en la boca:
- El comprimido para chupar se disuelve, y el fármaco se libera. Este proceso tiene lugar en unos 15 minutos.
- El fármaco es absorbido a través de la mucosa oral hacia la circulación sanguínea.
El hecho de usar el medicamento de dicha forma permite que se absorba rápidamente, lo que significa un alivio rápido del dolor irruptivo.
Determinación de la dosis correcta
Debería empezar a sentir alivio rápidamente mientras usa Abfentiq. No obstante, hasta que usted y su médico determinen la dosis que controla eficazmente el dolor irruptivo, es posible que no sienta un alivio suficiente del dolor 30 minutos después de haber empezado a utilizar una unidad de Abfentiq (15 minutos desde que acaba de utilizar el comprimido de Abfentiq). Si esto ocurriera, su médico le puede permitir utilizar un segundo comprimido de Abfentiq de la misma dosis para tratar un mismo episodio de dolor irruptivo.
No utilice una segunda unidad a menos que se lo indique su médico.
Nunca utilice más de dos unidades para tratar un solo episodio de dolor irruptivo.
Durante la determinación de la dosis correcta, es posible que necesite disponer de unidades de Abfentiq de distintas concentraciones en su domicilio. Sin embargo, conserve en casa únicamente las concentraciones de Abfentiq que necesite. Esto permite prevenir posibles confusiones y sobredosis. Consulte con el farmacéutico cómo desprenderse de las unidades de Abfentiq que no necesita.
Cuántas unidades se deben utilizar
Una vez haya determinado la dosis correcta con su médico, use 1 unidad para un episodio de dolor irruptivo. Consulte con su médico si su dosis correcta de Abfentiq no le alivia el dolor irruptivo a lo largo de varios episodios consecutivos de dolor irruptivo. Su médico decidirá si es preciso modificarle la dosis.
Debe comunicar enseguida a su médico si usa Abfentiq más de cuatro veces al día. En ese caso quizás crea conveniente cambiarle el medicamento para el dolor persistente (presente todo el tiempo). Una vez hecho, cuando se le haya controlado el dolor persistente, puede que el médico deba volver a cambiarle la dosis de Abfentiq. Para obtener mejores resultados, informe a su médico sobre el dolor que padece y sobre cómo Abfentiq está actuando. De este modo se podrá cambiar la dosis si resulta necesario.
Uso del medicamento
Apertura del envoltorio
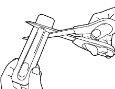
- Cada unidad de Abfentiq está sellada en su propio envoltorio blister.
No abra el envoltorio antes de tiempo.
- Sujete el envoltorio blister con el lado impreso opuesto a usted.
- Sujete el extremo corto del envoltorio blister.
- Coloque las tijeras cerca del extremo de la unidad Abfentiq y corte completamente el extremo largo (vea la ilustración).
- Separe la parte posterior impresa del envoltorio blister y extráigala
completamente del envoltorio.
 Saque la unidad de Abfentiq del envoltorio blister y acto seguido coloque
Saque la unidad de Abfentiq del envoltorio blister y acto seguido coloque
el comprimido de Abfentiq en la boca.
Uso de la unidad de Abfentiq
- Coloque el comprimido entre las mejillas y las encías.
- Con el aplicador, desplace contínuamente Abfentiq por la boca,
especialmente por las mejillas. Gire el aplicador a menudo.
 Para que el alivio sea más eficaz, debe acabarse totalmente la unidad
Para que el alivio sea más eficaz, debe acabarse totalmente la unidad
de Abfentiq en unos 15 minutos. Si la termina demasiado rápido, tragará más
medicamento y obtendrá menos alivio del dolor irruptivo.
- No muerda ni mastique la unidad de Abfentiq. Esto se traduciría en
menores niveles en sangre y menor alivio del dolor que si se utiliza según
se indica.
- Si por alguna razón no termina toda la unidad de Abfentiq cada vez que padece
dolor irruptivo, póngase en contacto con su médico.
Frecuencia de administración
Una vez que haya conseguido una dosis que le controle eficazmente el dolor, no utilice más de cuatro unidades de Abfentiq al día. Si cree que quizás necesita más de cuatro unidades de Abfentiq diarias, debe notificárselo de inmediato a su médico.
Cuántas unidades de Abfentiq debe usar
No utilice más de dos comprimidos para chupar de Abfentiq para tratar un solo episodio de dolor irruptivo.
Si usa más Abfentiq del que debe
Los efectos adversos más habituales si usa demasiado son somnolencia, mareos y náuseas.
- Si empieza a sentirse mareado, con ganas de vomitar o con mucho sueño antes de que el comprimido para chupar se haya disuelto completamente, retírelo de la boca y pida a otra persona de la casa que le ayude.
Un efecto adverso grave de Abfentiq es la respiración lenta y/o poco profunda. Esto puede ocurrir si la dosis de Abfentiq es demasiado elevada o si usted usa demasiado Abfentiq.
- Si esto ocurriera busque asistencia médica enseguida.
- En caso de sobredosis o ingestión accidental, consulte a su médico o farmacéutico o llame al Servicio de Información Toxicológica. Teléfono: 91 562 04 20, indicando el medicamento y la cantidad ingerida.
Qué hacer si un niño o un adulto usa accidentalmente Abfentiq
Si cree que alguien ha usado accidentalmente Abfentiq, busque asistencia médica enseguida. Intente mantener la persona despierta (llamándola por su nombre o sacudiéndola por el brazo o el hombro) hasta que llegue la asistencia médica.
Si olvidó usar Abfentiq
Si todavía persiste el dolor irruptivo, debe usar Abfentiq según le haya indicado su médico. Si el dolor irruptivo desaparece, no use más Abfentiq hasta que aparezca otro episodio de dolor irruptivo.
Si interrumpe el tratamiento con Abfentiq
No deje de usar Abfentiq sin consultarlo con su médico. No suelen aparecer efectos perceptibles si deja de usar Abfentiq. Siga usando su medicamento opiáceo habitual para tratar el dolor persistente (presente todo el tiempo) según le indique su médico.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, Abfentiq puede producir efectos adversos, aunque no todas las personas los sufran. Si nota algún efecto adverso, contacte con su médico.
Los efectos adversos más graves son respiración poco profunda, tensión arterial baja y shock.
Usted o su cuidador deben retirar la unidad de Abfentiq de la boca. Contacte con su médico inmediatamente y solicite ayuda urgente si usted experimenta alguno de los siguientes efectos adversos – es posible que necesite atención médica urgente:
- Si está muy somnoliento o tiene una respiración lenta o poco profunda.
- Dificultad para respirar o mareado, hinchazón de la lengua, labios o garganta que pueden ser los primeros signos de una reacción alérgica grave.
Nota para las personas que le cuiden:
Si observa que el paciente que usa Abfentiq tiene una respiración lenta y/o poco profunda o si le cuesta despertarle, tome INMEDIATAMENTE las siguientes medidas:
- Coja la unidad de Abfentiq por el aplicador, quítesela al paciente de la boca y manténgala fuera del alcance de los niños o de los animales de compañía hasta que la deseche.
- SOLICITE ASISTENCIA DE URGENCIA
- Mientras espera que llegue la asistencia de urgencia, si parece que la persona respira lentamente, incítela a respirar cada 5–10 segundos.
Si se siente excesivamente mareado, somnoliento o experimenta cualquier otro malestar mientras usa Abfentiq, retírese la unidad de Abfentiq de la boca utilizando el aplicador y deséchela según las instrucciones explicadas en este prospecto (ver sección 5). A continuación, póngase en contacto con su médico para que le dé nuevas instrucciones de uso de Abfentiq.
Efectos adversos muy frecuentes(pueden afectar a más de 1 de cada 10 pacientes):
- Vómitos, náuseas/malestar, estreñimiento, dolor estomacal (abdominal)
- Astenia (debilidad), Somnolencia, sedación, mareo. Dolor de cabeza.
- Falta de aliento.
Efectos adversos frecuentes(pueden afectar hasta 1 de cada 10 pacientes):
- Confusión, ansiedad, ver o escuchas cosas que no están (alucinaciones), depresión, cambios de humor.
- Sensación de malestar
- Ataque, espasmo muscular, sensación de vértigo o de mareo, pérdida de conciencia, sedación, sensación de hormigueo, entumecimiento, dificultad para coordinar movimientos, aumento o alteración de la sensibilidad al tacto, convulsiones (crisis epilépticas).
- Sequedad de boca, inflamación bucal, afecciones de la lengua (por ejemplo, sensación de ardor o úlceras), alteraciones del gusto.
- Gases, hinchazón abdominal, indigestión, disminución del apetito, pérdida de peso.
- Visión borrosa o doble.
- Sudoración, erupciones cutáneas, picor cutáneo.
- Dificultad para orinar
- Lesiones accidentales (por ejemplo, caídas).
- Malestar general.
Efectos adversos poco frecuentes(pueden afectar hasta 1 de cada 100 pacientes):
- Caries dental, parálisis del intestino, úlceras en la boca, sangrado de las encías.
- Coma, dificultad para hablar.
- Sueños anormales, sensación de indiferencia, pensamientos anormales, sensación excesiva de encontrarse bien.
- Dilatación de los vasos sanguíneos
- Urticaria
Frencuencia no conocida:
- Disminución de las encías, inflamación de las encías, pérdida dental, problemas respiratorios graves, rubor, sensación de mucho calor, diarrea, inflamación de brazos o piernas, fatiga.
- Dependencia de drogas (adicción)
- Abuso de drogas
- Delirio (los síntomas pueden consistir en una combinación de agitación, inquietud, desorientación, confusión, miedo, ver u oír cosas que realmente no existen, trastornos del sueño, pesadillas)
Mientras utiliza Abfentiq puede experimentar irritación, dolor y úlceras en el lugar de aplicación y sangrado de encías.
El tratamiento prolongado con fentanilo durante el embarazo puede causar síntomas de abstinencia en el recién nacido, que pueden ser potencialmente mortales (ver sección 2).
Comunicación de Efectos Adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de Medicamentos de Uso Humano: https://www.notificaram.es Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Abfentiq
Mantener este medicamento fuera de la vista y del alcance de los niños.
El medicamento analgésico de Abfentiq es muy potente y podría resultar potencialmente mortal para un niño si lo usa accidentalmente. Abfentiq debe mantenerse fuera del alcance y de la vista de los niños.
- No utilice este medicamento después de la fecha de caducidad que aparece en el envase blister y el cartonaje después de “CAD:”. La fecha de caducidad es el último día del mes que se indica.
- Conservar por debajo de 25ºC.
- Mantener Abfentiq siempre en su envoltorio blister hasta que usted esté preparado para usarlo. No utilice este medicamento si observa que el envoltorio blister está dañado o abierto antes de que usted esté listo para usarlo.
Los medicamentos no se deben tirar por los desagües ni a la basura. Deposite los envases y los medicamentos que no necesita en el Punto SIGRE de la farmacia. En caso de duda pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que no necesita. De esta forma, ayudará a proteger el medio ambiente.
- Cómo eliminar Abfentiq una vez usado
Las unidades parcialmente utilizadas de Abfentiq pueden contener todavía suficiente medicamento como para resultar nocivas o potencialmente mortales en un niño.
Incluso tanto si queda algo de medicamento como si no en el aplicador, el aplicador debe eliminarse apropiadamente, de la siguiente forma:
- Si no queda nada de medicamento, tire el aplicador a un contenedor de basura que esté fuera del alcance de los niños y animales de compañía.
- Si queda medicamento en el aplicador, ponga el comprimido debajo de un grifo de agua caliente para disolver los restos y a continuación tire el aplicador a un contenedor de basura que esté fuera del alcance de los niños y animales de compañía.
- Si no termina toda la unidad de Abfentiq y no puede disolver los restos de medicamento inmediatamente, ponga la unidad de Abfentiq fuera del alcance de los niños y animales de compañía hasta que disponga de tiempo para desechar la unidad de Abfentiq parcialmente empleada según se ha explicado.
- No tire por el W.C. las unidades de Abfentiq parcialmente empleadas, los aplicadores de Abfentiq ni el envoltorio blister.
6. Contenido del envase e información adicional
Composición de Abfentiq 200 400, 600, 800, 1200, 1600 microgramos
El principio activo es fentanilo. Cada comprimido para chupar contiene 200 400, 600, 800, 1200, 1600 microgramos de fentanilo (como citrato).
Los demás componentes son:
Comprimido para chupar:
Dextratos hidratados, ácido cítrico anhidro, hidrogenofosfato de sodio anhidro, sabor artificial de baya, estearato de magnesio.
Adhesivo comestible utilizado para pegar el comprimido al aplicador:
Almidón comestible a base de maíz modificado (E 1450), dextratos hidratados, agua.
Aplicador:
Resina ABS
Tinta alimentaria (E-133)
Aspecto del producto y contenido del envase
Abfentiq es un sistema para la administración de medicamentos directamente a través de la mucosa oral. Cada unidad de Abfentiq se compone de un medicamento sólido de color blanco unido a un aplicador.
La unidad normalmente es blanca, sin embargo, durante el almacenamiento puede adquirir un aspecto ligeramente moteado. Esto se debe a ligeros cambios en el aromatizante del producto y no afecta en modo alguno la acción del medicamento.
Abfentiq existe en 6 dosis diferentes: 200, 400, 600, 800, 1200 y 1600 microgramos. La dosis está marcada en el comprimido blanco, en el aplicador, en el blister y en el cartonaje, para garantizar que usted use el medicamento y dosis adecuados. Cada dosis está asociada a un color específico.
Cada envoltorio blister contiene una sola unidad de Abfentiq, suministrada en cajas de 3,6, 15 ó 30 unidades individuales de Abfentiq.
Puede que solamente estén comercializados algunos tamaños de envases.
Titular de la autorización de comercialización y responsable de la fabricación
Titular de la autorización de comercialización:
FERRER INTERNACIONAL, S.A.
Gran Vía Carlos III, 94
08028 Barcelona (España)
Responsable de la fabricación:
PRASFARMA, S.L.
c/ Sant Joan 11-15.
08560 Manlleu (Barcelona)
España
Representante local:
FERRER FARMA, S.A.
Av. Diagonal, 549 5ª planta
08029 Barcelona (España)
Fecha de la última revisión de este prospecto: Febrero 2022
La información detallada y actualizada de este medicamento está disponible en la página Web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) http://www.aemps.gob.es/
- País de registro
- Precio medio en farmacia206.41 EUR
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a ABFENTIQ 600 MICROGRAMOS COMPRIMIDOS PARA CHUPAR EFGForma farmacéutica: PARCHE TRANSDERMICO, 12 MCG/HPrincipio activo: fentaniloFabricante: Aristo Pharma Iberia S.L.Requiere recetaForma farmacéutica: COMPRIMIDO BUCAL/PARA CHUPAR, 1200 microgramosPrincipio activo: fentaniloFabricante: Ferrer Internacional S.A.Requiere recetaForma farmacéutica: COMPRIMIDO BUCAL/PARA CHUPAR, 1600 microgramosPrincipio activo: fentaniloFabricante: Ferrer Internacional S.A.Requiere receta
Médicos online para ABFENTIQ 600 MICROGRAMOS COMPRIMIDOS PARA CHUPAR EFG
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de ABFENTIQ 600 MICROGRAMOS COMPRIMIDOS PARA CHUPAR EFG, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes















