
SOMAVERT 30 mg PÓ E SOLVENTE PARA SOLUÇÃO INJETÁVEL

Pergunte a um médico sobre a prescrição de SOMAVERT 30 mg PÓ E SOLVENTE PARA SOLUÇÃO INJETÁVEL

Como usar SOMAVERT 30 mg PÓ E SOLVENTE PARA SOLUÇÃO INJETÁVEL
Introdução
Prospecto: informação para o utilizador
SOMAVERT 10 mg pó e dissolvente para solução injetável
SOMAVERT 15 mg pó e dissolvente para solução injetável
SOMAVERT 20 mg pó e dissolvente para solução injetável
SOMAVERT 25mg pó e dissolvente para solução injetável
SOMAVERT 30mg pó e dissolvente para solução injetável
pegvisomanto
Leia todo o prospecto atentamente antes de começar a usar este medicamento, porque contém informações importantes para si.
- Conserva este prospecto, porque pode ter que voltar a lê-lo.
- Se tiver alguma dúvida, consulte o seu médico, farmacêutico ou enfermeiro.
- Este medicamento foi prescrito apenas para si, e não deve dá-lo a outras pessoas, mesmo que tenham os mesmos sintomas que si, porque pode prejudicá-las.
- Se experimentar efeitos adversos, consulte o seu médico, farmacêutico ou enfermeiro, mesmo que se trate de efeitos adversos que não aparecem neste prospecto. Ver secção 4.
Conteúdo do prospecto
- O que é SOMAVERT e para que é utilizado
- O que precisa saber antes de começar a usar SOMAVERT
- Como usar SOMAVERT
- Efeitos adversos possíveis
- Conservação de SOMAVERT
- Conteúdo do envase e informação adicional
1. O que é SOMAVERT e para que é utilizado
SOMAVERT é utilizado para o tratamento da acromegalia, um distúrbio hormonal resultante do aumento da secreção da hormona do crescimento (HC) e do IGF-I (fatores de crescimento tipo insulina), e caracteriza-se por sobre-crescimento dos ossos, engrossamento dos tecidos moles, doença do coração e distúrbios relacionados.
O princípio ativo de SOMAVERT, pegvisomanto, é conhecido como um antagonista do receptor da hormona do crescimento. Estas substâncias reduzem a ação da HC e os níveis de IGF-I que circulam no sangue.
2. O que precisa saber antes de começar a usar SOMAVERT
Não use SOMAVERT
- Se for alérgico a pegvisomanto ou a algum dos outros componentes deste medicamento (incluídos na secção 6).
Advertências e precauções
Consulte o seu médico, farmacêutico ou enfermeiro antes de começar a usar SOMAVERT.
- Se notar distúrbios na visão ou dores de cabeça, deve dizer-lho ao seu médico imediatamente.
- O seu médico ou enfermeira controlará os níveis de IGF-I (fatores de crescimento tipo insulina) que circulam no sangue e, se necessário, ajustará a dose de SOMAVERT.
- O seu médico também deve controlar o seu adenoma (tumor benigno).
- O seu médico realizará testes de função hepática antes de começar e durante o tratamento com SOMAVERT. Se os resultados desses testes não forem normais, o seu médico discutirá as opções de tratamento consigo. Uma vez que o tratamento comece, o seu médico ou enfermeira controlará os níveis de enzimas hepáticas no sangue cada 4-6 semanas durante os primeiros 6 meses de tratamento com SOMAVERT. A administração de SOMAVERT deve ser suspensa se persistirem os sintomas da doença hepática.
- Se for diabético, o seu médico pode precisar ajustar a quantidade de insulina ou de outros medicamentos que esteja a utilizar.
- A fertilidade nas pacientes pode aumentar à medida que melhora a doença. Não se recomenda utilizar este medicamento em mulheres grávidas e deve aconselhar as mulheres em idade fértil a utilizar um método anticonceptivo. Ver também o apartado sobre Gravidez que aparece mais adiante.
Outros medicamentos e SOMAVERT
Deve dizer ao seu médico se utilizou anteriormente outro medicamento para o tratamento da acromegalia ou algum medicamento para o tratamento da diabetes.
Informa ao seu médico ou farmacêutico se está a utilizar ou utilizou recentemente qualquer outro medicamento. Como parte do seu tratamento, pode ser tratado com outros medicamentos. É importante que continue a utilizar todos os medicamentos, incluindo SOMAVERT, a menos que receba outra indicação por parte do seu médico, farmacêutico ou enfermeiro.
Gravidez, lactação e fertilidade
Não se recomenda utilizar SOMAVERT em mulheres grávidas. Se for uma mulher em idade fértil, deve utilizar um método anticonceptivo durante o tratamento.
Não se sabe se o pegvisomanto passa para o leite materno. Não deve amamentar enquanto estiver a tomar SOMAVERT, a menos que tenha discutido isso com o seu médico.
Se estiver grávida ou em período de lactação, acredita que possa estar grávida ou tem intenção de ficar grávida, consulte o seu médico ou farmacêutico antes de utilizar este medicamento.
Condução e uso de máquinas
Não foram realizados estudos sobre os efeitos na capacidade para conduzir e utilizar máquinas.
SOMAVERT contém sódio
Este medicamento contém menos de 1 mmol de sódio (23 mg) por dose; isto é, é essencialmente “isento de sódio”.
3. Como usar SOMAVERT
Siga exatamente as instruções de administração deste medicamento indicadas pelo seu médico ou farmacêutico. Em caso de dúvida, consulte novamente o seu médico ou farmacêutico.
O seu médico administrará por via subcutânea (debaixo da pele) uma dose inicial de 80 mg de pegvisomanto. Depois, a dose diária habitual é de 10 mg de pegvisomanto administrada por injeção subcutânea (debaixo da pele).
Cada 4-6 semanas, o seu médico realizará os ajustes de dose necessários aumentando a dose em 5 mg de pegvisomanto por dia, de acordo com os seus níveis no sangue do já mencionado IGF-I, com o fim de obter uma resposta terapêutica óptima.
Forma e via de administração
SOMAVERT é injetado sob a pele. A injeção pode ser feita por si ou por outra pessoa, como o seu médico ou ajudante. Deve seguir as instruções detalhadas sobre o processo de injeção, que se incluem no final deste prospecto. Deve continuar a injetar este medicamento durante todo o tempo que o seu médico indicar.
Este medicamento deve ser dissolvido antes do uso. A injeção não deve ser misturada na mesma seringa ou frasco com outro medicamento.
O tecido gorduroso da pele pode aumentar no local da injeção. Para evitar isso, utilize pontos de injeção ligeiramente diferentes cada vez, tal como se descreve no passo 2 da secção deste prospecto “Instruções para a preparação e administração de uma injeção de SOMAVERT”. Assim, dará tempo à pele e à zona sob a pele para se recuperarem entre uma injeção e outra antes de voltar a injetar no mesmo local.
Se tiver a impressão de que o efeito deste medicamento é demasiado forte ou demasiado fraco, fale com o seu médico, farmacêutico ou enfermeiro.
Se injetar mais SOMAVERT do que deve
Se acidentalmente se injetou mais quantidade de SOMAVERT do que o seu médico disse, não é provável que isso seja grave, mas deve indicá-lo imediatamente ao seu médico, farmacêutico ou enfermeiro.
Se esquecer de usar SOMAVERT
Se esquecer de se injetar uma dose, deve injetar a dose seguinte assim que se lembrar e deve continuar a injetar SOMAVERT tal como foi prescrito pelo seu médico. Não se injete uma dose dupla para compensar as doses esquecidas.
Se tiver alguma outra dúvida sobre o uso deste medicamento, pergunte ao seu médico, farmacêutico ou enfermeiro.
4. Efeitos adversos possíveis
Como todos os medicamentos, este medicamento pode produzir efeitos adversos, embora nem todas as pessoas os sofram.
Foram notificadas reações alérgicas (anafilácticas) de leves a graves em alguns pacientes que usam SOMAVERT. Os sintomas de uma reação alérgica grave podem incluir um ou mais dos seguintes sintomas: inchaço do rosto, da língua, dos lábios ou da garganta; assobios ou dificuldade para respirar (espasmo da laringe); erupção generalizada da pele, urticária ou comichão; ou tontura. Entre em contacto imediatamente com o seu médico se apresentar algum desses sintomas.
Muito frequentes: podem afetar mais de 1 em cada 10 pessoas:
- Dor de cabeça.
- Diarreia.
- Dor nas articulações.
Frequentes: podem afetar até 1 em cada 10 pessoas:
- Dificuldade para respirar.
- Aumentos nos níveis das substâncias que determinam a função do fígado. Podem ser vistos nos resultados dos análises de sangue.
- Sangue na urina.
- Aumento da tensão arterial.
- Prisão de ventre, mal-estar, sensação de estar doente, sensação de estar inchado, dispepsia, flatulência.
- Tontura, sonolência, tremor incontrolado, diminuição do sentido do tacto.
- Equimoses ou sangramento no local da injeção, dor ou inchaço no local da injeção, aumento do tecido gorduroso sob a pele no local da injeção, inchaço dos membros, fraqueza, febre.
- Sudorese, comichão, erupção, tendência para equimoses.
- Dor nos músculos, artrite.
- Aumento do colesterol no sangue, aumento de peso, aumento da glicose no sangue, descida da glicose no sangue.
- Sintomas gripais, fadiga.
- Sonhos anormais.
- Dor nos olhos.
Pouco frequentes: podem afetar até 1 em cada 100 pessoas:
- Reação alérgica após a administração (febre, erupção, comichão e, em casos graves, dificuldade para respirar, inchaço rápido da pele, que requerem atenção médica urgente). Podem ocorrer imediatamente ou vários dias após a administração.
- Proteínas na urina, aumento da quantidade de urina, problemas nos rins.
- Ausência de interesse, sensação de confusão, aumento da libido, ataques de pânico, perda de memória, dificuldades para dormir.
- Redução de plaquetas no sangue, aumento ou redução de leucócitos no sangue, tendência para sangrar.
- Sensação anormal, alteração na cicatrização.
- Pesadez nos olhos, problemas no ouvido interno.
- Inchaço do rosto, secura da pele, sudorese noturna, rubor da pele (eritema), comichão e urticária elevada na pele.
- Aumento de substâncias gordas no sangue, aumento do apetite.
- Seca da boca, aumento da salivação, problemas dentários, hemorroides.
- Sentido do gosto anormal, enxaquecas.
Não conhecidos: não se pode estimar a frequência a partir dos dados disponíveis
- Irritabilidade.
- Dificuldade para respirar grave (laringoespasmo).
- Inchaço rápido da pele, do tecido subcutâneo e do revestimento interno (mucosa) dos órgãos (angioedema).
Aproximadamente 17% dos pacientes desenvolverão anticorpos contra a hormona do crescimento durante o tratamento. Parece que os anticorpos não afetam a ação deste medicamento.
Comunicação de efeitos adversos
Se experimentar qualquer tipo de efeito adverso, consulte o seu médico, farmacêutico ou enfermeiro, mesmo que se trate de possíveis efeitos adversos que não aparecem neste prospecto. Também pode comunicá-los directamente através do sistema nacional de notificação incluído no Apêndice V. Mediante a comunicação de efeitos adversos, pode contribuir para fornecer mais informações sobre a segurança deste medicamento.
5. Conservação de SOMAVERT
Mantenha este medicamento fora da vista e do alcance das crianças.
Não utilize este medicamento após a data de validade que aparece nos frascos e no envase após EXP. A data de validade é o último dia do mês que se indica.
Conservar o(s) frasco(s) de pó em frigorífico (entre 2°C e 8°C) no(s) seu(s) embalagem(s) para protegê-los da luz. Não congelar.
O(s) embalagem(s) que contém(ém) o(s) frasco(s) de pó de SOMAVERT pode(m) ser conservado(s) a temperatura ambiente até um máximo de 25°C durante um período único de até 30 dias. Escreva a data de validade no embalagem, incluindo o dia/mês/ano (até 30 dias a partir da data de extração do frigorífico). O(s) frasco(s) deve(m) estar protegido(s) da luz. Não volte a colocar este medicamento no frigorífico.
Elimine este medicamento se não o utilizar antes da nova data de validade ou da data de validade impressa no embalagem, o que ocorrer primeiro.
Conservar a(s) seringa(s) pré-carregada(s) a temperatura inferior a 30ºC ou conservar no frigorífico (entre 2ºC e 8ºC). Não congelar.
Após a preparação da solução de SOMAVERT, esta deve ser utilizada imediatamente.
Não utilize este medicamento se observar que a solução está turva ou contém partículas.
Os medicamentos não devem ser deitados fora nos esgotos nem na lixeira. Pergunte ao seu farmacêutico como se livrar dos embalagens e dos medicamentos que já não precisa. Dessa forma, ajudará a proteger o meio ambiente.
6. Conteúdo do frasco e informação adicional
Composição de SOMAVERT
- O princípio ativo é pegvisomanto.
- SOMAVERT 10 mg: um frasco de pó contém 10 mg de pegvisomanto. Após a reconstituição com 1 ml de solvente, 1 ml de solução contém 10 mg de pegvisomanto.
- SOMAVERT 15 mg: um frasco de pó contém 15 mg de pegvisomanto. Após a reconstituição com 1 ml de solvente, 1 ml de solução contém 15 mg de pegvisomanto.
- SOMAVERT 20 mg: um frasco de pó contém 20 mg de pegvisomanto. Após a reconstituição com 1 ml de solvente, 1 ml de solução contém 20 mg de pegvisomanto.
- SOMAVERT 25 mg: um frasco de pó contém 25 mg de pegvisomanto. Após a reconstituição com 1 ml de solvente, 1 ml de solução contém 25 mg de pegvisomanto.
- SOMAVERT 30 mg: um frasco de pó contém 30 mg de pegvisomanto. Após a reconstituição com 1 ml de solvente, 1 ml de solução contém 30 mg de pegvisomanto.
- Os outros componentes são glicina, manitol (E-421), hidrogenofosfato de sódio anidro, dihidrogenofosfato de sódio monohidratado (ver seção 2 “SOMAVERT contém sódio”).
- O solvente é água para preparações injetáveis.
Aspecto do produto e conteúdo do frasco
SOMAVERT é apresentado sob a forma de pó e solvente para injeção (em um frasco de 10 mg, 15 mg, 20 mg, 25 mg ou 30 mg de pegvisomanto e 1 ml de solvente em uma seringa pré-carregada). Tamanhos de embalagem de 1 e/ou 30. Pode ser que apenas alguns tamanhos de embalagens sejam comercializados.O pó é de cor branca e o solvente é transparente e incolor.
Título da autorização de comercialização e responsável pela fabricação:
Título da autorização de comercialização
Pfizer Europe MA EEIG
Boulevard de la Plaine 17
1050 Bruxelas
Bélgica
Responsável pela fabricação
Pfizer Manufacturing Belgium NV
Rijksweg 12
2870 Puurs-Sint-Amands
Bélgica
Pode solicitar mais informações sobre este medicamento dirigindo-se ao representante local do título da autorização de comercialização:
Bélgica/Bélgica/Bélgica Luxemburgo/Luxemburgo Pfizer NV/SA Tel: +32 (0)2 554 62 11 | Lituânia Pfizer Luxembourg SARL filial em Lituânia Tel: +370 5 251 4000 |
?????? ??????? ?????????? ????, ???? ???????? ???.: +359 2 970 4333 | Hungria Pfizer Kft. Tel.: + 36 1 488 37 00 |
República Tcheca Pfizer, spol. s r.o. Tel: +420 283 004 111 | Malta Vivian Corporation Ltd. Tel: +356 21344610 |
Dinamarca Pfizer ApS Tlf.: +45 44 20 11 00 | Países Baixos Pfizer bv Tel: +31 (0)800 63 34 636 |
Alemanha PFIZER PHARMA GmbH Tel: +49 (0)30 550055-51000 | Noruega Pfizer AS Tlf: +47 67 52 61 00 |
Estônia Pfizer Luxembourg SARL filial na Estônia Tel: +372 666 7500 | Áustria Pfizer Corporation Austria Ges.m.b.H. Tel: +43 (0)1 521 15-0 |
Grécia Pfizer ?????? ????? ?????: +30 210 6785800 | Polônia Pfizer Polska Sp. z o.o. Tel.: +48 22 335 61 00 |
Espanha Pfizer, S.L. Tel: +34 91 490 99 00 | Portugal Laboratórios Pfizer, Lda. Tel: +351 21 423 5500 |
França Pfizer Tél: +33 (0)1 58 07 34 40 | Romênia Pfizer Romania S.R.L. Tel: +40 (0) 21 207 28 00 |
Croácia Pfizer Croatia d.o.o. Tel: + 385 1 3908 777 | Eslovênia Pfizer Luxembourg SARL Pfizer, filial para consultoria em atividades farmacêuticas, Liubliana Tel: +386 (0)1 52 11 400 |
Irlanda Pfizer Healthcare Ireland Unlimited Company Tel: 1800 633 363 (gratuito) Tel: +44 (0)1304 616161 | República Eslovaca Pfizer Luxembourg SARL, filial Tel: + 421 2 3355 5500 |
Islândia Icepharma hf. Sími: +354 540 8000 | Finlândia Pfizer Oy Puh/Tel: +358 (0)9 430 040 |
Itália Pfizer S.r.l. Tel: +39 06 33 18 21 | Suécia Pfizer AB Tel: +46 (0)8 550 520 00 |
Chipre Pfizer ?????? ????? (filial no Chipre) ?????: +357 22817690 | |
Letônia Pfizer Luxembourg SARL filial na Letônia Tel: + 371 670 35 775 | |
Data da última revisão deste folheto: 02/2025.
Outras fontes de informação
A informação detalhada sobre este medicamento está disponível no site da Agência Europeia de Medicamentos: https://www.ema.europa.eu. Também existem links para outros sites sobre doenças raras e medicamentos órfãos.
INSTRUÇÕES DE USO
SOMAVERT pó em frasco com solvente em uma seringa pré-carregada
pegvisomanto para solução injetável
Apenas para injeção subcutânea
Frasco de dose única
SOMAVERT é apresentado em um frasco como um bloco de pó branco. Deve misturar SOMAVERT com um líquido (solvente) antes de poder usá-lo.
O líquido é apresentado em uma seringa pré-carregada com a etiqueta “Solvente para SOMAVERT”.
Não misture SOMAVERT com nenhum outro líquido.
É importante que não tente administrar a si mesmo ou a outra pessoa uma injeção sem ter recebido treinamento de seu profissional de saúde.
Conservar o(s) estojo(s) dos frascos de pó na geladeira entre 2°C e 8°C e longe da luz solar direta.
O(s) embalagem(ns) que contêm o(s) frasco(s) de pó de SOMAVERT pode(m) ser conservado(s) a temperatura ambiente até um máximo de 25°C durante um período único de até 30 dias. Escreva a data de validade no embalagem, incluindo o dia/mês/ano (até 30 dias a partir da data de retirada da geladeira). O(s) frasco(s) deve(m) estar protegido(s) da luz. Não volte a colocar este medicamento na geladeira.
Descarte este medicamento se não o usar antes da nova data de validade ou da data de validade impressa no embalagem, o que ocorrer primeiro.
A seringa pré-carregada de solvente pode ser conservada a temperatura ambiente. Manter fora do alcance das crianças.
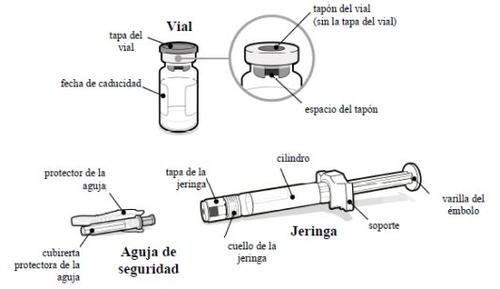
- O que você precisa
Um embalagem de SOMAVERT com:
- Um frasco de SOMAVERT pó
- Uma seringa pré-carregada com solvente
- Uma agulha de segurança
Também precisa:
- Uma torunda de algodão
- Um algodão com álcool
- Um contenedor de objetos pontiagudos apropriado
- Preparação
Antes de começar:
- Misture SOMAVERT com o solvente apenas quando estiver preparado para injetar a dose.
- Retire um único embalagem de SOMAVERT da geladeira e deixe que atinja a temperatura ambiente de forma natural em um local seguro.
- Lave as mãos com água e sabão, e seque-as bem.
- Abra o envoltório da seringa e da agulha de segurança para que seja mais fácil pegar cada elemento enquanto se prepara para a injeção.
- Não use a seringa ou o frasco se:
- estão danificados ou defeituosos;
- a data de validade foi ultrapassada;
- a seringa foi congelada, mesmo se foi descongelada posteriormente (apenas a seringa).
- Escolha uma zona de injeção
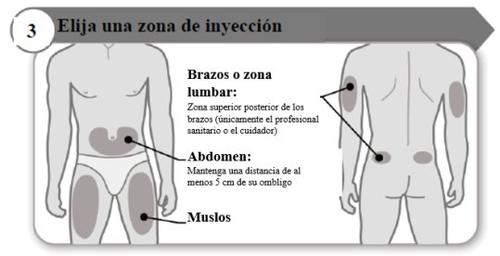
- Escolha um local diferente dentro de cada área para a injeção.
- Evite as zonas ósseas, vermelhas, dolorosas ou duras, ou que tenham hematomas, cicatrizes ou doenças de pele.
- Limpe a zona de injeção com o algodão com álcool como seu profissional de saúde indicou.
- Espere até que a zona de injeção seque.
- Retire a tampa do frasco
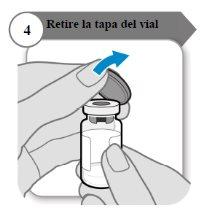
- Retire a tampa do frasco.
- Descarte a tampa; não é necessário novamente.
Precaução:Não deixe que nada toque o batoque do frasco.
- Retire a tampa da seringa
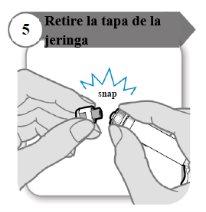
- Desprenda a tampa da seringa. Pode ser que você precise de mais força do que o esperado.
- Descarte a tampa da seringa; não é necessário novamente.
- Mantenha a seringa na posição vertical para evitar vazamentos.
Precaução:Não deixe que o extremo da seringa toque nada uma vez que você tenha retirado a tampa.
- Coloque a agulha de segurança
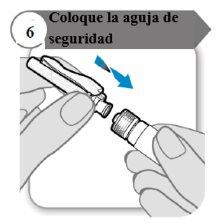
- Coloque a agulha de segurança na seringa girando firmemente tanto quanto possível.
- Retire a cobertura protetora da agulha
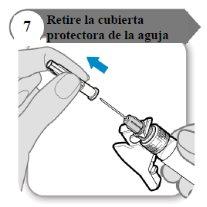
- Dobre o protetor da agulha para fora, afastando-o da cobertura protetora da agulha.
- Com cuidado, puxe a cobertura protetora da agulha diretamente para fora.
- Descarte a cobertura protetora da agulha; não é necessário novamente.
Precaução:Não deixe que a agulha toque nada.
- Insira a agulha
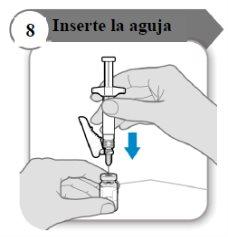
- Empurre a agulha através do centro do batoque do frasco como indicado.
- Segure a seringa enquanto a agulha estiver inserida no batoque do frasco para evitar que a agulha se dobre.
- Adicione o líquido
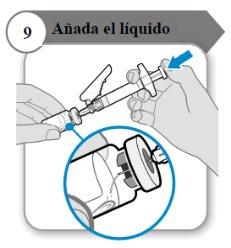
- Incline o frasco e a seringa formando um ângulo como indicado.
- Empurre a varinha do êmbolo lentamenteaté que todo o líquido esteja dentro do frasco.
- Precaução:Certifique-se de que o líquido não caia diretamente sobre o pó, pois isso forma espuma. A espuma torna o medicamento inutilizável.
- Não retire a agulha ainda.
- Gire o frasco
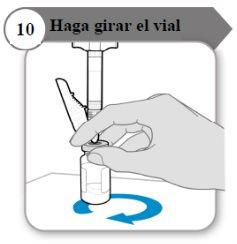
- Segure a seringa e o frasco com uma mão como indicado.
- Gire o líquido suavemente deslizando o frasco com um movimento circular sobre uma superfície plana.
- Continue girando o líquido até que todo o pó esteja completamente dissolvido.
Nota:Isso pode levar até 5 minutos.
- Examine o medicamento
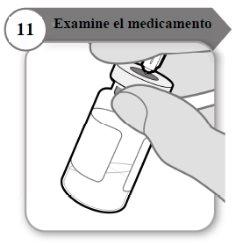
- Com a agulha ainda inserida no frasco, inspecione o medicamento atentamente. Ele deve ser transparente e sem partículas.
- Não o use se:
- o medicamento está turvo ou escuro;
- o medicamento tem algum cor;
- contém partículas ou há uma camada de espuma no frasco.
- Reposicione a agulha
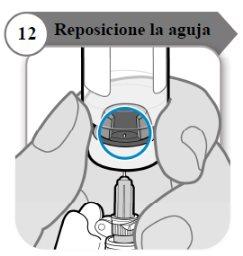
- Gire o frasco de modo que você possa ver o espaço no batoque do frasco, como indicado.
- Puxe a agulha para baixo de modo que a ponta da agulha esteja no ponto mais baixo no líquido. Isso ajudará a retirar tanto líquido quanto possível.
- Verifique se a varinha do êmbolo não se moveu. Se moveu, empurre para voltar a introduzir por completo na seringa. Isso garante que todo o ar tenha saído da seringa antes de retirar a dose.
- Retire a dose
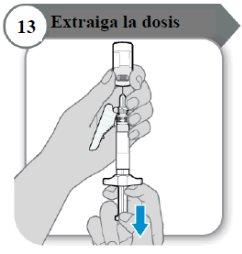
- Puxe a varinha do êmbolo devagar para retirar tanta quantidade de medicamento do frasco quanto possível.
Nota:Se você observar ar na seringa, pressione o cilindro da seringa para que as bolhas se desloquem para cima, e então empurre as bolhas devagar para o frasco.
- Retire a agulha do frasco.
- Insira a agulha
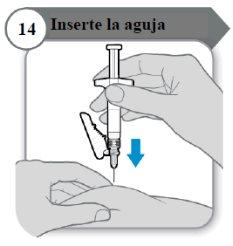
- Com cuidado, puxe a pele na zona de injeção.
- Insira a agulha por completo na pele puxada.
- Injete o medicamento
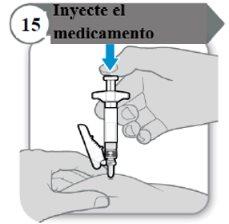
- Empurre a varinha do êmbolo para baixo devagar até que a seringa esteja vazia.
Nota:Certifique-se de que a agulha esteja inserida por completo.
- Solte a pele puxada e retire a agulha em linha reta.
- Asegure a agulha
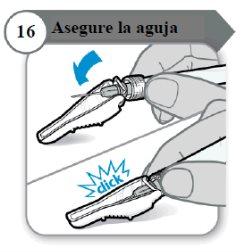
- Dobre o protetor da agulha sobre a agulha.
- Com cuidado, pressione contra uma superfície dura para fechar o protetor da agulha.
Nota:Você ouvirá um clique quando o protetor da agulha se fechar.
- Descarte
- A seringa e a agulha não devem ser reutilizadas NUNCA. Descarte a agulha e a seringa como seu médico, enfermeiro ou farmacêutico indicou e de acordo com as diretrizes de saúde locais e a legislação de segurança.
- Depois da injeção
- Se necessário, pressione levemente com uma torunda de algodão limpo na zona de injeção.
- Não esfregue a zona.
PERGUNTAS E RESPOSTAS
O que devo fazer se algo tocou acidentalmente o batoque do frasco?
- Limpe o batoque do frasco com um algodão com álcool novo e deixe que seque por completo. Se não for capaz de limpar o batoque, não use o frasco.
O que devo fazer com a seringa se ela caiu?
- Não a use, mesmo se parecer que não está danificada. Descarte a seringa da mesma forma que descarta uma seringa usada. Você precisará de outra seringa.
Quantas vezes posso inserir com segurança a agulha no batoque do frasco?
- Apenas uma vez. Retirar e reinsertar a agulha aumenta consideravelmente o risco de dano à agulha e pode deixá-la cega. Isso pode causar desconforto e aumentar o risco de dano à pele e infecção. Também existe o risco de que parte do medicamento seja perdida.
Está bem agitar o frasco se o pó não se dissolve?
- Não, nunca agite o frasco. As sacudidas podem inutilizar o medicamento e formar espuma. O pó pode levar alguns minutos para se dissolver completamente, então continue movendo o frasco suavemente com movimentos circulares até que o líquido seja completamente transparente.
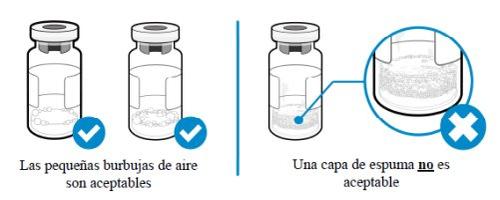
Como posso saber se há espuma no frasco?
- A espuma aparece como uma massa de pequenas bolhas que flutuam formando uma camada sobre o líquido. Não injete SOMAVERT se houver espuma.
Como posso evitar que o medicamento forme espuma?
- Empurre o êmbolo muito devagar de modo que o líquido flua suavemente para dentro do frasco. Não deixe o líquido cair diretamente sobre o pó, pois isso forma espuma. Essa técnica também reduzirá o tempo necessário para misturar o medicamento e permitirá retirar mais medicamento.
Posso ver um pouco de ar na seringa. Está bem?
- As pequenas bolhas de ar no líquido são normais e a injeção é segura. No entanto, é possível aspirar acidentalmente um pouco de ar dentro da seringa, que deve ser eliminado antes da injeção. As bolhas ou espaços de ar que flutuam sobre o líquido devem ser expulsos para o frasco.
Por que não posso retirar todo o medicamento do frasco?
- A forma do frasco faz com que uma pequena quantidade do medicamento fique no frasco. Isso é normal. Para garantir que apenas uma pequena quantidade do medicamento fique no frasco, certifique-se de que a ponta da agulha esteja introduzida dentro do frasco tanto quanto possível quando retirar a dose.
O que devo fazer se tiver alguma dúvida sobre o medicamento?
- Todas as perguntas devem ser dirigidas a um médico, enfermeiro ou farmacêutico com experiência com SOMAVERT.
- País de registo
- Substância ativa
- Requer receita médicaSim
- Fabricante
- Esta informação é apenas para referência e não constitui aconselhamento médico. Consulte sempre um médico antes de tomar qualquer medicamento. A Oladoctor não se responsabiliza por decisões médicas baseadas neste conteúdo.
- Alternativas a SOMAVERT 30 mg PÓ E SOLVENTE PARA SOLUÇÃO INJETÁVELForma farmacêutica: INJETÁVEL, 10 mgSubstância ativa: pegvisomantFabricante: Pfizer Europe Ma EeigRequer receita médicaForma farmacêutica: INJETÁVEL, 15 mgSubstância ativa: pegvisomantFabricante: Pfizer Europe Ma EeigRequer receita médicaForma farmacêutica: INJETÁVEL, 20 mgSubstância ativa: pegvisomantFabricante: Pfizer Europe Ma EeigRequer receita médica
Alternativas a SOMAVERT 30 mg PÓ E SOLVENTE PARA SOLUÇÃO INJETÁVEL noutros países
As melhores alternativas com o mesmo princípio ativo e efeito terapêutico.
Alternativa a SOMAVERT 30 mg PÓ E SOLVENTE PARA SOLUÇÃO INJETÁVEL em Ukraine
Médicos online para SOMAVERT 30 mg PÓ E SOLVENTE PARA SOLUÇÃO INJETÁVEL
Avaliação de posologia, efeitos secundários, interações, contraindicações e renovação da receita de SOMAVERT 30 mg PÓ E SOLVENTE PARA SOLUÇÃO INJETÁVEL – sujeita a avaliação médica e regras locais.














