
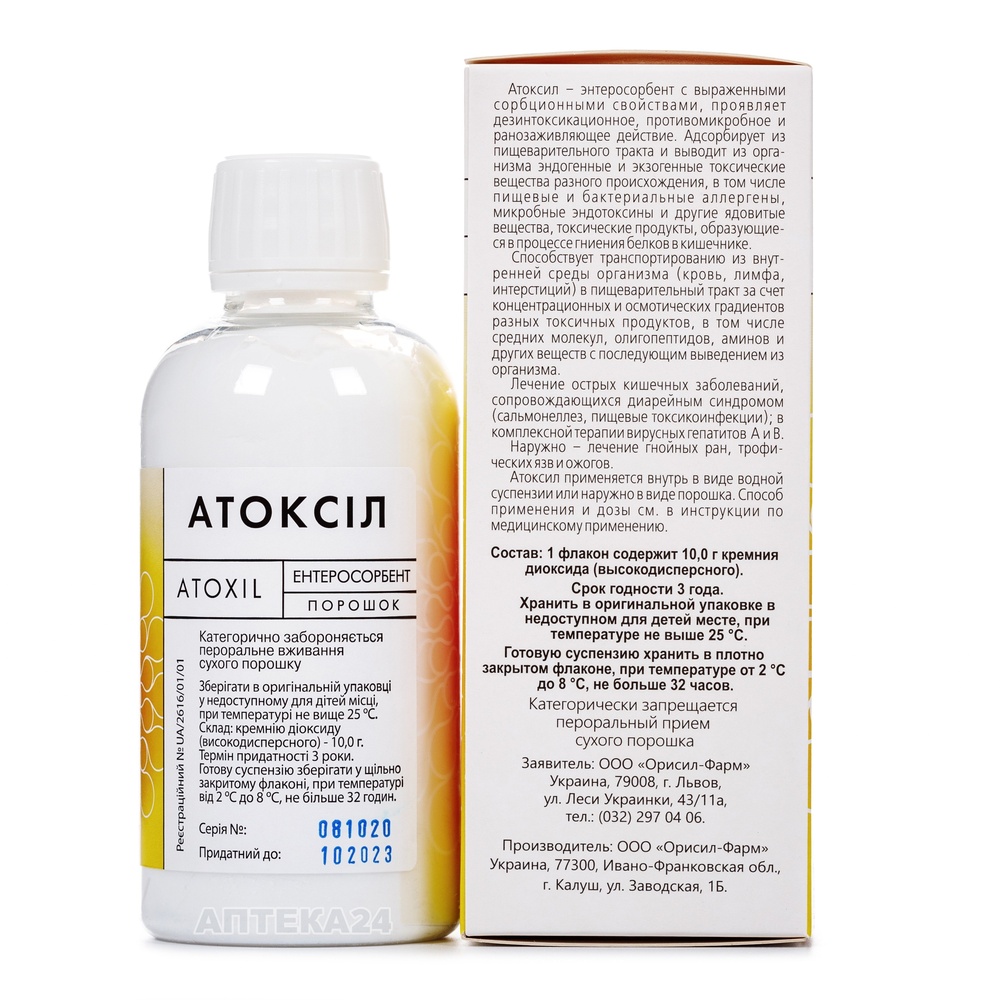
ATOKSIL

Ask a doctor about a prescription for ATOKSIL

How to use ATOKSIL
INSTRUCTIONS FOR MEDICAL USE OF THE MEDICINAL PRODUCT NOVONORM®(NOVONORM®)
Composition
active substance: repaglinide;
1 tablet contains:
1 mg tablet - 1 mg of repaglinide;
excipients: microcrystalline cellulose (E 460), calcium hydrophosphate, corn starch, potassium polacrylate, povidone K25, glycerin 85%, magnesium stearate, meglumine, poloxamer 188, yellow iron oxide (E 172) - dye for 1 mg tablets;
2 mg tablet - 2 mg of repaglinide;
excipients: microcrystalline cellulose (E 460), calcium hydrophosphate, corn starch, potassium polacrylate, povidone K25, glycerin 85%, magnesium stearate, meglumine, poloxamer 188, red iron oxide (E 172) - dye for 2 mg tablets.
Pharmaceutical Form
Tablets.
Main Physico-Chemical Properties
1 mg tablets: yellow, round, biconvex, one side marked with the Novo Nordisk company symbol;
2 mg tablets: brown-pink, round, biconvex, one side marked with the Novo Nordisk company symbol.
Pharmacotherapeutic Group
Antidiabetic drugs. Other hypoglycemic drugs, excluding insulins.
ATC Code A10B X02.
Pharmacological Properties
Pharmacodynamics
Mechanism of Action
Repaglinide is a fast-acting oral insulin secretion stimulator. Repaglinide quickly lowers blood glucose levels by stimulating insulin secretion by the pancreas, and the effect of the drug depends on the number of functioning beta-cells in the gland.
Repaglinide blocks ATP-dependent potassium channels in the beta-cell membrane with a special protein that is different from other insulin secretion stimulants. This causes depolarization of beta-cells and leads to the opening of calcium channels, which increases the entry of calcium ions into the cell, stimulating insulin secretion.
Effects Related to Pharmacodynamics
In patients with type 2 diabetes, an increase in insulin concentration in the blood occurs within 30 minutes after oral administration of repaglinide. This reduces blood glucose levels throughout the entire period of food absorption. The increased insulin level persists only during food intake. The concentration of repaglinide in the blood plasma quickly decreases, and its low level is noted in patients with type 2 diabetes within 4 hours after administration.
Clinical Efficacy and Safety
After taking 0.5 to 4 mg of repaglinide by patients with type 2 diabetes, a dose-dependent decrease in glucose levels was shown. Based on the results of clinical studies, it is recommended to take repaglinide during main meals (preprandial administration). The drug is usually taken 15 minutes before eating, but the time of administration can vary from immediately before eating to 30 minutes before eating (i.e., preprandially with two-, three-, and four-time meals). If a patient misses a meal (or has an additional meal), they should accordingly miss (or add) a dose of the drug.
Pharmacokinetics
Absorption
Repaglinide is quickly absorbed from the gastrointestinal tract, leading to a rapid increase in the concentration of the drug in the blood plasma. The peak concentration of the drug in the blood plasma occurs within 1 hour after administration. After reaching the peak, the concentration of the drug in the blood plasma quickly decreases. The pharmacokinetics of repaglinide is characterized by an average absolute bioavailability of 63% (coefficient of variation 11%). Taking repaglinide immediately before eating, 15 minutes before eating, or 30 minutes before eating, or on an empty stomach, does not significantly affect its pharmacokinetics. In clinical studies, a high (60%) variability in the concentration of repaglinide in the blood plasma of different patients was noted; in the same patient, its level varies from low to moderate (35%). Since the selection of the repaglinide dose is based on the patient's clinical response, the high variability in different patients does not affect the effectiveness of the drug.
Distribution
The pharmacokinetics of repaglinide is characterized by a low volume of distribution - 30 L (which corresponds to distribution in intracellular fluid), and repaglinide is easily bound (more than 98%) to blood plasma proteins.
Elimination
Repaglinide is quickly eliminated from the blood within 4-6 hours. The half-life is approximately 1 hour. Repaglinide is almost completely metabolized. Its metabolites do not cause a clinically significant hypoglycemic effect.
Repaglinide and its metabolites are excreted mainly with bile. A small fraction (less than 8%) of the administered dose is found in the urine in the form of metabolites. Less than 1% of the administered dose is found in the feces.
Special Patient Groups
Increased Effect of Repaglinide in Patients with Liver Dysfunction and in Elderly Patients with Type 2 Diabetes
AUC (mean error) after a single dose of 2 mg (4 mg for patients with liver dysfunction) was 31.4 ng/mL/h (28.3) in healthy volunteers, 304.9 ng/mL/h (228.0) in patients with liver dysfunction, and 117.9 ng/mL/h (83.8) in elderly patients with type 2 diabetes.
After 5 Days of Treatment with Repaglinide (2 mg 3 Times a Day)
in patients with severe renal impairment (creatinine clearance 20-39 mL/min), the results showed a statistically significant doubling of exposure (AUC) and half-life (t1/2) compared to patients with normal renal function.
Children
Data is not available.
Data on Preclinical Safety
Preclinical data based on studies of pharmacological safety, toxicity of repeated doses, genotoxicity, and carcinogenicity did not reveal any hazard to humans.
In animal studies, it has been shown that repaglinide does not have a teratogenic effect. Embryotoxicity, limb defects were found in the fetuses and newborn rats born to rats that were given high doses of the drug during the last stage of pregnancy and during lactation. Repaglinide was found in the milk of experimental animals.
Clinical Characteristics
Indications
Type 2 diabetes (non-insulin-dependent diabetes), when it is not possible to achieve satisfactory control over blood glucose levels through diet, weight loss, and physical exercise. The use of repaglinide in combination with metformin is also indicated for patients with type 2 diabetes who have not achieved satisfactory glycemic control with metformin alone.
Treatment should be started as an addition to diet or physical exercise to reduce postprandial blood glucose levels.
Contraindications
- Hypersensitivity to repaglinide or to any component of the NovoNorm®drug.
- Type 1 diabetes (insulin-dependent diabetes), C-peptide-negative diabetes.
- Diabetic ketoacidosis with or without coma.
- Pregnancy or breastfeeding.
- Severe liver function disorders.
- Concomitant use with gemfibrozil (see the section "Interaction with other medicinal products and other types of interactions").
Interaction with Other Medicinal Products and Other Types of Interactions
As is known, a number of drugs can affect the metabolism of repaglinide. The possibility of such an interaction should be taken into account by the doctor when prescribing the drug.
According to in vitro data, the metabolism of repaglinide mainly involves the CYP2C8 and CYP3A4 enzymes. Studies with the participation of healthy volunteers showed that the most important enzyme in the metabolism of repaglinide is CYP2C8, while CYP3A4 plays a minor role. However, blocking CYP2C8 leads to an increase in the relative contribution of CYP3A4. Accordingly, the metabolism and clearance of repaglinide may change when using drugs that inhibit or induce the synthesis of cytochrome P450 enzymes. Particular caution should be exercised when using repaglinide simultaneously with CYP2C8 and CYP3A4 inhibitors. According to in vitro data, repaglinide is actively transported into liver cells with the participation of a protein that transports organic anions (OATP1B1 - organic anion transporting protein). Drugs that inhibit OATP1B1 (e.g., cyclosporin) may also potentially increase the concentration of repaglinide in the blood plasma.
Drugs that may enhance and/or prolong the hypoglycemic effect of repaglinide.
Gemfibrozil, trimethoprim, rifampicin, ketoconazole, itraconazole, clarithromycin, cyclosporin, deferasirox, clopidogrel, other antidiabetic drugs, monoamine oxidase inhibitors, non-selective beta-blockers, angiotensin-converting enzyme inhibitors, salicylates, non-steroidal anti-inflammatory drugs, octreotide, anabolic steroids, alcohol.
After simultaneous administration of 2 times a day 600 mg of gemfibrozil (an inhibitor of CYP2C8 and OATP1B1) and repaglinide (a single dose of 0.25 mg), the value of the area under the curve "repaglinide concentration - time" (AUC) in healthy volunteers increased 8.1 times, and its maximum concentration (Cmax) in the blood - 2.4 times. The half-life (t1/2) of repaglinide in the blood increased from 1.3 hours to 3.7 hours, which may enhance and prolong the hypoglycemic effect of repaglinide. When using gemfibrozil, the concentration of repaglinide in the blood plasma increased 28.6 times during 7 hours. Simultaneous use of gemfibrozil and repaglinide is contraindicated (see the section "Contraindications").
No drug interaction was found between fenofibrate and repaglinide.
After simultaneous administration of 2 times a day 160 mg of trimethoprim (a weak inhibitor of CYP2C8) and repaglinide (a single dose of 0.25 mg), the value of the area under the curve "repaglinide concentration - time" (AUC) increased 1.6 times, its Cmax - 1.4 times, and t1/2 - 1.2 times; at the same time, no statistically significant effect on blood glucose levels was found. Since the safety of this combination has not been established for repaglinide at a dose above 0.25 mg, and trimethoprim at a dose of 320 mg, simultaneous administration of these drugs should be carried out with caution. If necessary, treatment with a combination of these drugs should be carried out with careful monitoring of blood glucose levels and the patient's clinical condition.
Rifampicin, a potent inducer of CYP3A4 and CYP2C8, acts as an inducer and inhibitor of repaglinide metabolism. After 7 days of rifampicin administration at a dose of 600 mg with the addition of repaglinide (a single dose of 4 mg) on the seventh day, the value of the area under the curve "repaglinide concentration - time" (AUC) decreased by 50% (the result of combined induction and inhibition). If repaglinide was taken 24 hours after the last dose of rifampicin, the value of the area under the curve "repaglinide concentration - time" (AUC) decreased by 80% (the result of induction). When treating with rifampicin and repaglinide simultaneously, it is necessary to select a dose of the latter based on careful monitoring of blood glucose levels at such times: at the start of rifampicin administration (acute inhibition), after several days of rifampicin administration (combined induction and inhibition), after stopping rifampicin administration (only induction), and 2 weeks after stopping rifampicin administration, when its inductive effect has passed. It is not excluded that other inducers, such as phenytoin, carbamazepine, phenobarbital, and St. John's wort, may have a similar effect.
The effect of ketoconazole (a potent competitive inhibitor of CYP3A4) on the pharmacokinetics of repaglinide was studied in healthy volunteers. When taking ketoconazole 2 times a day at a dose of 200 mg simultaneously with repaglinide (a single dose of 4 mg), the value of the area under the curve "repaglinide concentration - time" (AUC) and Cmax increased 1.2 times; at the same time, the glucose concentration profile changed by less than 8%.
The simultaneous use of 100 mg of itraconazole (an inhibitor of CYP3A4) was also studied in healthy volunteers. It was shown that in this case, the value of the area under the curve "repaglinide concentration - time" (AUC) increased 1.4 times. No significant changes in blood glucose levels were found in the examined patients.
When simultaneously using 250 mg of clarithromycin (an inhibitor of CYP3A4) and repaglinide in healthy volunteers, the value of the area under the curve "repaglinide concentration - time" (AUC) increased 1.4 times, and Cmax - 1.7 times. At the same time, the value of the area under the curve "insulin concentration in blood serum - time" (AUC) increased 1.5 times (Cmax - 1.6 times). The mechanism of this interaction has not been elucidated.
A study with the participation of healthy volunteers who took repaglinide (a single dose of 0.25 mg) showed that cyclosporin (repeated doses of 100 mg), which is an inhibitor of CYP3A4 and OATP1B1, increased the maximum concentration of repaglinide 1.8 times and the area under the curve (AUC) 2.5 times. Since the safety of this combination has not been established for repaglinide at a dose above 0.25 mg, simultaneous administration of these drugs should be avoided. If necessary, treatment with a combination of these drugs should be carried out with careful monitoring of blood glucose levels and the patient's clinical condition.
Simultaneous administration of deferasirox (a moderate inhibitor of CYP2C8 and CYP3A4) at a dose of 30 mg/kg body weight per day for 4 days and repaglinide (a single dose of 0.5 mg) in healthy volunteers led to an increase in the duration of action of repaglinide (area under the curve - AUC) 2.3 times (90% confidence interval [2.03-2.63]) and maximum concentration (Cmax) 1.6 times (90% confidence interval [1.42-1.84]), which led to a slight (but statistically significant) decrease in blood glucose levels. Since the interaction of these drugs at doses for repaglinide above 0.5 mg has not been established, it is worth avoiding their simultaneous administration. If necessary, treatment with a combination of these drugs should be carried out with careful monitoring of blood glucose levels and the patient's clinical condition (see the section "Special Instructions").
Simultaneous administration of the CYP2C8 inhibitor clopidogrel (loading dose of 300 mg) increased the value of the area under the curve "repaglinide concentration - time" (AUC0-∞) 5.1 times, and repeated administration (daily dose of 75 mg) increased the value of the area under the curve "repaglinide concentration - time" (AUC0-∞) 3.9 times. A slight statistically significant decrease in blood glucose levels was observed. If repaglinide and clopidogrel are used simultaneously, careful monitoring of the patient's clinical condition and blood glucose levels is necessary (see the section "Special Instructions").
Beta-blockers can mask symptoms of hypoglycemia.
Simultaneous administration of cimetidine, nifedipine, estrogen, or simvastatin (substrates of CYP3A4) with repaglinide does not significantly affect the values of its pharmacokinetic parameters.
Studies of drug interactions with the participation of healthy volunteers showed that repaglinide does not significantly affect the pharmacokinetics of digoxin, theophylline, and warfarin. Therefore, when using these drugs simultaneously with repaglinide, dose adjustment is not required.
Drugs that May Weaken the Hypoglycemic Effect of Repaglinide
Oral contraceptives, rifampicin, barbiturates, carbamazepine, thiazides, corticosteroids, danazol, thyroid hormones, and sympathomimetics. When prescribing or canceling these drugs in patients taking repaglinide, it is necessary to carefully monitor changes in blood glucose levels. During treatment with repaglinide, along with other drugs that, like repaglinide, are mainly excreted with bile, any possible interactions should be considered.
Children
No studies of drug interactions with the participation of children and adolescents have been conducted.
Special Instructions
Repaglinide should be prescribed in case of unsatisfactory control over blood glucose levels through diet, physical exercise, and weight loss.
If a patient who has achieved stable glycemic control with oral hypoglycemic drugs is subjected to stress (fever, trauma, infectious diseases, or surgical interventions), disruptions to this control may occur. In such cases, the need to stop taking repaglinide and temporarily switch to insulin administration may arise.
Hypoglycemia
Repaglinide, like other insulin secretion stimulants, can cause hypoglycemia.
Combination with Insulin Secretion Stimulants
In many patients with prolonged use of oral hypoglycemic drugs, their hypoglycemic effect decreases. This may be associated with the progression of diabetes severity or a decrease in the body's response to the drug. This phenomenon is called secondary failure, which should be distinguished from primary failure, in which the patient does not respond to the drug taken for the first time. Before diagnosing secondary failure, it is necessary to try to change the dose and also check the patient's adherence to dietary and physical activity recommendations.
Combination Therapy with Neutral Protamine Hagedorn (NPH) Insulin or Thiazolidinediones
Studies on combination therapy with NPH insulin or thiazolidinediones have been conducted. However, an assessment of the risk/benefit ratio for other types of combination therapy is necessary.
Combination Therapy with Metformin
When using combination therapy with metformin, the risk of hypoglycemia increases.
Acute Coronary Syndrome
Treatment with repaglinide may be associated with an increased risk of developing acute coronary syndrome (e.g., myocardial infarction); see the sections "Side Effects" and "Pharmacodynamic Properties".
Combination Therapy
Patients who are taking drugs that affect the metabolism of repaglinide should use the drug with caution or not take it at all (see the section "Interaction with other medicinal products and other types of interactions"). If combination therapy is necessary, careful monitoring of blood glucose levels and the patient's clinical condition is necessary.
Special Patient Groups
Elderly Patients
Clinical studies with the participation of patients over 75 years old have not been conducted.
Renal Impairment
Renal impairment does not affect repaglinide. 8% of the repaglinide dose is excreted by the kidneys, and the total clearance of repaglinide is decreased in renal impairment. Since patients with diabetes complicated by renal impairment have increased insulin sensitivity, caution should be exercised when selecting the dose of the drug.
Hepatic Impairment
Clinical studies with the participation of patients with hepatic impairment have not been conducted.
Weak and Emaciated Patients
The selection of the initial and maintenance doses, as well as the titration of the drug, should be carried out with particular care in weak and emaciated patients to prevent the development of hypoglycemia.
Patients Taking Other Oral Hypoglycemic Drugs
Patients can be immediately transferred from other oral hypoglycemic drugs to repaglinide. However, the exact ratio of doses between repaglinide and other oral hypoglycemic drugs has not been established. The maximum recommended initial dose for patients transferred to repaglinide is 1 mg before main meals.
If the level of glucose in the blood is not effectively controlled by metformin, repaglinide can be added to it. In this case, the dose of metformin should be left unchanged, and repaglinide should be prescribed simultaneously. The starting dose of repaglinide is 0.5 mg. Dose titration should be carried out according to the glucose level in the blood.
Use During Pregnancy or Breastfeeding
Pregnancy
Studies on the use of repaglinide in pregnant women have not been conducted. The use of repaglinide should be avoided during pregnancy.
Breastfeeding
Studies on the use of repaglinide in breastfeeding women have not been conducted. Repaglinide should not be used in breastfeeding women.
Fertility
Information on reproductive toxicity studies in animals is provided in the section "Preclinical Safety Data".
Ability to Affect Reaction Speed When Driving Vehicles or Operating Other Mechanisms
NovoNorm®does not have a direct effect on reaction speed when driving vehicles or operating other mechanisms, but it can lead to hypoglycemia.
Patients should be advised to take precautions to prevent hypoglycemia while driving vehicles. This is especially important for those with weakened symptoms of hypoglycemia or those who frequently experience episodes of hypoglycemia. In these cases, the feasibility of driving vehicles in general should be assessed.
Method of Administration and Dosage
Dosage
Repaglinide is taken orally before each main meal (i.e., preprandially), and the dose should be individually selected to optimize glycemic control. In addition to self-monitoring of blood glucose and/or urine by the patient, the doctor should periodically monitor the concentration of glucose in the blood of patients to determine the minimum effective dose of the drug. The level of glycosylated hemoglobin is also an informative indicator when monitoring the patient's response to treatment. Periodic monitoring is necessary to detect inadequate reduction in blood glucose levels when using the maximum recommended dose (i.e., primary failure) and to detect the absence of a sufficient decrease in blood glucose levels after an effective initial treatment period (i.e., secondary failure).
Initial Dose
The selection of the drug dose is carried out by the doctor according to the patient's response. The recommended initial dose is 0.5 mg. Dose selection should begin after 1-2 weeks, based on the glucose level in the blood as an indicator of the response to treatment. If the patient is transferred to repaglinide from another oral hypoglycemic drug, the recommended initial dose is 1 mg.
Maintenance Therapy
The maximum recommended single dose before main meals is 4 mg. The maximum daily dose should not exceed 16 mg.
Method of Administration
The drug should be taken orally, usually 15 minutes before the start of a meal, but the time of administration can vary from immediately before eating to 30 minutes before eating (i.e., preprandially with two-, three-, and four-time meals). If a patient misses a meal (or has an additional meal), they should accordingly miss (or add) a dose of the drug.
Children
Repaglinide is not recommended for use in children (under 18 years old) due to insufficient data on the safety and efficacy of the drug in this patient group.
Overdose
During 6 weeks, repaglinide was administered in increasing doses from 4 to 20 mg 4 times a day. No safety concerns were recorded. Since in this study, the possibility of hypoglycemia was prevented due to the high caloric content of food, relative overdose could lead to pronounced hypoglycemia and the development of symptoms such as dizziness, sweating, tremors, and headache. In the event of such symptoms, appropriate measures should be taken to normalize blood glucose levels (carbohydrate intake). In more severe hypoglycemia, accompanied by seizures, loss of consciousness, or coma, glucose should be administered intravenously.
Side Effects
Safety Data
The most common side effects are those related to changes in blood glucose levels, such as hypoglycemia. The frequency of occurrence of such reactions depends on individual patient characteristics: eating habits, drug dose, level of physical activity, and stress.
List of Undesirable Reactions
Based on the experience of using repaglinide and other hypoglycemic drugs, the following side effects can occur: often (≥1/100 to <1/10); uncommon (≥1/1000 to <1/100); rare (≥1/10000 to <1/1000); very rare (<1/10000); frequency not established (cannot be estimated based on available data).
Immune System Disorders
Very rare: allergic reactions.*
Metabolic Disorders
Often: hypoglycemia.
Frequency not established: hypoglycemia with loss of consciousness and hypoglycemic coma.
Eye Disorders
Very rare: refractive disorders.*
Cardiovascular Disorders
Rare: cardiovascular diseases.
Gastrointestinal Disorders
Often: abdominal pain, diarrhea.
Very rare: vomiting, constipation.
Frequency not established: nausea.
Hepatobiliary Disorders
Very rare: liver function disorders, increased liver enzyme levels.*
Skin and Subcutaneous Tissue Disorders
Frequency not established: hypersensitivity reactions.*
Description of Individual Side Effects
Allergic reactions.
Generalized hypersensitivity reactions (e.g., anaphylactic reactions) or immune reactions, such as vasculitis.
Refractive disorders.
Fluctuations in blood glucose levels can lead to temporary visual disturbances, especially at the beginning of treatment. Such disturbances have been reported in only a very small number of patients after starting treatment with repaglinide. In clinical studies, it was not found that these cases led to discontinuation of repaglinide treatment.
Liver function disorders, increased liver enzyme levels.
During treatment with repaglinide, isolated cases of increased liver enzyme levels have been noted. In most cases, this was moderate and short-term, and very few patients were forced to stop treatment with the drug due to increased liver enzyme levels. Severe liver function disorders have been observed very rarely.
Hypersensitivity reactions.
A hypersensitivity reaction to the drug can manifest as redness, itching, rash, and urticaria. Due to the different chemical structure, it is not possible to suspect a possible cross-allergic reaction with sulfonylurea drugs.
Shelf Life
5 years.
Do not take the drug after the expiration date indicated on the packaging.
Storage Conditions
Store in the original packaging at a temperature not exceeding 25 °C.
Store in a place inaccessible to children.
Packaging
15 tablets in a blister pack with aluminum foil on both sides; 2 or 6 blister packs in a cardboard box.
Release Category
By prescription.
Manufacturer/Applicant
Novo Nordisk A/S, Denmark/Novo Nordisk A/S, Denmark.
Location of the Manufacturer and Address of the Place of Business/Location of the Applicant
Novo Alle, Bagsvaerd, 2880, Denmark.
- Country of registration
- Prescription requiredNo
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to ATOKSILDosage form: powder, 3 gActive substance: diosmectiteManufacturer: Приватне акціонерне товариство "Лекхім-ХарківPrescription not requiredDosage form: powder, 3 g/3.76 g, 3.76 g powder in sachetActive substance: diosmectitePrescription not requiredDosage form: powder, 3 gActive substance: diosmectiteManufacturer: ТОВ "ТернофармPrescription not required
Online doctors for ATOKSIL
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for ATOKSIL – subject to medical assessment and local rules.














