ZYPADHERA 405 mg POWDER AND SOLVENT FOR PROLONGED-RELEASE INJECTABLE SUSPENSION


How to use ZYPADHERA 405 mg POWDER AND SOLVENT FOR PROLONGED-RELEASE INJECTABLE SUSPENSION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
ZYPADHERA 210 mg powder and solvent for prolonged-release injectable suspension
ZYPADHERA 300 mg powder and solvent for prolonged-release injectable suspension
ZYPADHERA 405 mg powder and solvent for prolonged-release injectable suspension
Olanzapine
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or nurse.
- If you experience any side effects, talk to your doctor or nurse, even if they are not listed in this leaflet. See section 4.
Contents of the pack:
- What ZYPADHERA is and what it is used for
- What you need to know before you use ZYPADHERA
- How to use ZYPADHERA
- Possible side effects
- Storage of ZYPADHERA
- Contents of the pack and further information
1. What ZYPADHERA is and what it is used for
ZYPADHERA contains olanzapine as the active substance. ZYPADHERA belongs to a group of medicines called antipsychotics and is used to treat schizophrenia - an illness with symptoms such as hearing, seeing, or feeling things that do not exist, mistaken beliefs, unusual suspiciousness, and withdrawal. People with this illness may also feel depressed, anxious, or tense.
ZYPADHERA is indicated in adult patients who have been previously stabilized during treatment with oral olanzapine.
2. What you need to know before you use ZYPADHERA
Do not use ZYPADHERA:
- if you are allergic to olanzapine or any of the other ingredients of this medicine (listed in section 6). An allergic reactionmay appear as a skin rash, itching, swelling of the face, lips, or difficulty breathing. If you have ever suffered from any of these symptoms, please inform your doctor or nurse.
- if you have previously been diagnosed with eye problems, such as certain types of glaucoma (increased pressure in the eye).
Warnings and precautions
Talk to your doctor or nurse before starting to use ZYPADHERA.
- A rare but serious reaction may occur after you receive each injection.Sometimes, ZYPADHERA may enter the bloodstream too quickly. If this happens to you, you may experience some of the symptoms listed below after each injection. In some cases, these symptoms can cause loss of consciousness.
• | excessive drowsiness | • | dizziness | |
• | confusion | • | disorientation | |
• | irritability | • | anxiety | |
• | aggression |
| ||
• | difficulty speaking | • | blood pressure | |
• | difficulty walking | weakness | ||
• | seizures |
|
These symptoms usually disappear within 24 to 72 hours after the injection. After each injection, you should remain under observation at your healthcare center for at least 3 hours in case you experience any of the symptoms mentioned above.
Although it is unlikely, you may experience these symptoms more than 3 hours after the injection. If this happens to you, contact your doctor or nurse immediately. Due to this risk, you should not drive vehicles or operate machinery for the rest of the day after each injection.
- If you feel dizzy or faint after the injection, inform your doctor or nurse. You may need to lie down until you feel better. The doctor or nurse may want to take your blood pressure and check your pulse.
- ZYPADHERA is not recommended for use in elderly patients with dementia(confusion or memory loss) as it may cause serious side effects.
- In very rare cases, medicines of this type can cause unusual movements, mainly in the face or tongue, or a combination of fever, rapid breathing, sweating, muscle stiffness, and drowsiness or sleepiness. If this happens to you after receiving ZYPADHERA, inform your doctor or nurse immediately.
- A weight gain has been observed in patients taking ZYPADHERA. You and your doctor should check your weight regularly. If necessary, your doctor can help you plan a diet or consider referring you to a nutritionist.
- High levels of sugar and fats (triglycerides and cholesterol) in the blood have been observed in patients using ZYPADHERA. Your doctor should perform blood tests to check your blood sugar and fat levels before you start using ZYPADHERA and regularly during treatment.
- Tell your doctor if you or a family member have a history of blood clots, as medicines of this type have been associated with the formation of blood clots.
Tell your doctor as soon as possible if you suffer from any of the following conditions:
- Stroke or "mini" stroke (transient symptoms of stroke)
- Parkinson's disease
- Prostate problems
- Intestinal obstruction (paralytic ileus)
- Liver or kidney disease
- Blood disorders
- Recent myocardial infarction, coronary disease, sick sinus syndrome (abnormal heart rhythms), unstable angina, or low blood pressure.
- Diabetes
- Seizures
- If you think you may have loss of salts due to prolonged and intense diarrhea and vomiting or due to the use of diuretic medications (water pills)
As a routine precaution, it is recommended to measure blood pressure periodically in patients over 65 years old.
It is not recommended to start treatment with ZYPADHERA if you are over 75 years old.
Children and adolescents
Patients under 18 years old should not use ZYPADHERA.
Other medicines and ZYPADHERA
Tell your doctor if you are taking, have recently taken, or might take any other medicines.
Especially, tell your doctor if you are taking:
- medicines for Parkinson's disease.
- carbamazepine (an antiepileptic and mood stabilizer), fluvoxamine (an antidepressant), or ciprofloxacin (an antibiotic) - your dose of ZYPADHERA may need to be adjusted.
If you are already taking antidepressants, medicines to relieve anxiety, or to help you sleep (tranquilizers), you may feel more drowsy if you take ZYPADHERA.
Using ZYPADHERA with alcohol
You should avoid all alcohol consumption if you have been administered ZYPADHERA, as it may cause drowsiness when combined with alcohol.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor before receiving this injection.
You should not receive this injection if you are breastfeeding, as small amounts of olanzapine may pass into breast milk.
The following symptoms may occur in newborn babies of mothers who have been treated with ZYPADHERA in the last trimester of pregnancy (last three months of pregnancy): tremors, stiffness and/or muscle weakness, drowsiness, agitation, breathing problems, and difficulty feeding. If your baby develops any of these symptoms, you should contact your doctor.
Driving and using machines
Do not drive or use machines for the rest of the day after each injection.
ZYPADHERA contains sodium
Once reconstituted, this medicine contains less than 1 mmol of sodium (23 mg) per vial; i.e., it is essentially "sodium-free".
3. How to use ZYPADHERA
Your doctor will decide the amount of ZYPADHERA you need and how often you need to receive an injection. ZYPADHERA is administered in doses of 150 mg to 300 mg every 2 weeks or 300 mg to 405 mg every 4 weeks.
ZYPADHERA is presented as a powder that your doctor or nurse will reconstitute to create a suspension that will then be injected into the muscle of the buttock.
If you use more ZYPADHERA than you should
This medicine will be administered under medical supervision. Therefore, it is unlikely that you will receive too much.
Patients who have received more olanzapine than they should have also experienced the following symptoms:
- rapid heartbeat, agitation/aggression, speech problems, unusual movements (especially in the face or tongue), and decreased levels of consciousness.
Other symptoms may include:
- acute confusion, seizures (epilepsy), coma, a combination of fever, rapid breathing, sweating, muscle stiffness, and drowsiness or sleepiness, slow breathing, aspiration, high or low blood pressure, and abnormal heart rhythms.
Contact your doctor or hospital immediately if you experience any of the symptoms described above.
If you forget to use ZYPADHERA
Do not stop your treatment just because you start to feel better. It is important that you continue to receive ZYPADHERA for the entire time indicated by your doctor.
If you miss your appointment for your injection, you should contact your doctor to schedule the next injection as soon as possible.
If you have any further questions about the use of this medicine, ask your doctor or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Contact your doctor immediately if you have:
- excessive drowsiness, dizziness, confusion, disorientation, difficulty speaking, difficulty walking, muscle stiffness or agitation, weakness, irritability, aggression, anxiety, increased blood pressure, or seizures, and may cause loss of consciousness. These signs and symptoms may be due to the fact that ZYPADHERA may enter the bloodstream too quickly (a frequent adverse effect that may affect up to 1 in 10 people);
- unusual movements (a frequent adverse effect that may affect up to 1 in 10 people), especially of the face or tongue;
- blood clots in the veins (an uncommon adverse effect that may affect up to 1 in 100 people), especially in the legs (symptoms include swelling, pain, and redness in the leg), which can travel through the blood to the lungs, causing chest pain and difficulty breathing. If you experience any of these symptoms, seek medical attention immediately.
- a combination of fever, rapid breathing, sweating, muscle stiffness, and drowsiness or sleepiness (the frequency cannot be estimated from the available data)
Other frequent adverse effects (may affect up to 1 in 10 people) with ZYPADHERA include drowsiness and pain at the injection site.
Uncommon adverse effects with ZYPADHERA (may affect up to 1 in 1,000 people) include infection at the injection site.
Adverse effects that are listed below have been observed when olanzapine is administered orally, but may occur after administration of ZYPADHERA.
Very common adverse effects (may affect more than 1 in 10 people) include weight gain and increased prolactin levels in the blood. In the early stages of treatment, some people may feel dizzy or faint (with slower heartbeats), especially when standing up from a lying or sitting position. This feeling usually disappears on its own, but if it does not, consult your doctor.
Common adverse effects (may affect up to 1 in 10 people) include changes in the levels of certain blood cells, fats in the blood, and temporary increases in liver enzymes at the start of treatment; increased blood sugar and urine levels; increased uric acid and creatine phosphokinase levels in the blood; increased appetite; dizziness; agitation; tremors; unusual movements (dyskinesias); constipation; dry mouth; skin rash; loss of strength; excessive tiredness; fluid retention causing swelling in the hands, ankles, or feet; fever; joint pain; and sexual dysfunctions such as decreased libido in men and women or erectile dysfunction in men.
Uncommon adverse effects (may affect up to 1 in 100 people) include hypersensitivity (e.g., inflammation of the mouth and throat, itching; skin rash); diabetes or worsening of diabetes, occasionally related to ketoacidosis (acetone in blood and urine) or coma; seizures, in most cases related to a history of seizures (epilepsy); muscle stiffness or spasms (including eye movements); restless legs syndrome; speech problems; stuttering; slow heartbeat; sensitivity to sunlight; nosebleeds; abdominal distension; excessive salivation; memory loss or forgetfulness; urinary incontinence; loss of urination ability; hair loss; absence or decrease of menstrual periods; and changes in the breast gland in men and women, such as abnormal milk production or growth.
Rare adverse effects (may affect up to 1 in 1,000 people) include decreased body temperature; abnormal heart rhythms; sudden death of unknown origin; pancreatitis, which causes severe stomach pain and vomiting; and liver disease, which appears as yellowing of the skin and the white part of the eyes.
Very rare adverse effects include severe allergic reactions such as drug reaction with eosinophilia and systemic symptoms (DRESS). Initially, DRESS appears with symptoms similar to the flu, with a rash on the face, and later with an extensive rash, fever, swollen lymph nodes, elevated liver enzymes observed in blood tests, and an increase in a type of white blood cell in the blood (eosinophilia).
During treatment with olanzapine, elderly patients with dementia may experience stroke, pneumonia, urinary incontinence, falls, excessive tiredness, visual hallucinations, increased body temperature, skin redness, and difficulty walking. Some cases of death have been observed in this particular group of patients.
Olanzapine administered orally may worsen symptoms in patients with Parkinson's disease.
Reporting of side effects
If you experience any side effects, talk to your doctor or nurse, even if they are not listed in this leaflet. You can also report them directly through the national reporting system included in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of ZYPADHERA
Keep this medicine out of the sight and reach of children.
Do not administer the injection after the expiry date stated on the packaging.
Do not refrigerate or freeze.
The chemical and physical stability of the suspension in the vials has been demonstrated for 24 hours at 20 - 25°C. From a microbiological point of view, the medicine should be administered immediately. If not, the storage times and conditions before use are the responsibility of the healthcare professional and normally should not exceed 24 hours at 20 - 25°C. Do not use this product if you notice discoloration or other visible signs of deterioration.
If the medicine is not used immediately, it should be shaken vigorously to achieve resuspension. Once the suspension is withdrawn from the vial to the syringe, it should be used immediately.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of the packaging and any unused medicine. This will help protect the environment.
6. Container Contents and Additional Information
ZYPADHERA Composition
The active ingredientis olanzapine.
ZYPADHERA 210 mg: Each vial contains olanzapine pamoate monohydrate, equivalent to 210 mg of olanzapine.
ZYPADHERA 300 mg: Each vial contains olanzapine pamoate monohydrate, equivalent to 300 mg of olanzapine.
ZYPADHERA 405 mg: Each vial contains olanzapine pamoate monohydrate, equivalent to 405 mg of olanzapine.
Once reconstituted, each milliliter of the suspension contains 150 mg/ml of olanzapine.
The solvent componentsare sodium carmellose, mannitol, polysorbate 80, water for injectable preparations, hydrochloric acid, and sodium hydroxide.
Product Appearance and Container Contents
ZYPADHERA powder for prolonged-release injectable suspension is presented as a yellow powder in a transparent glass vial. Your doctor or nurse will reconstitute it into a suspension that will be administered by injection using the contents of the ZYPADHERA solvent vial, which is presented as a clear, colorless, or pale yellow solution in a transparent glass vial.
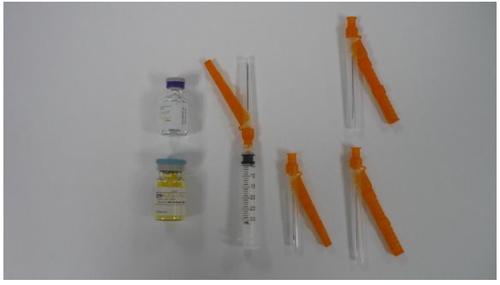
ZYPADHERA is a powder and solvent for prolonged-release injectable suspension. Each container contains a vial of powder for prolonged-release injectable suspension, a 3 ml vial of solvent, a syringe with a 19-gauge, 38 mm safety needle attached, and three separate safety needles; one 19-gauge, 38 mm needle and two 19-gauge, 50 mm needles.
Marketing Authorization Holder
CHEPLAPHARM Registration GmbH, Weilerstr. 5e, 79540 Lörrach, Germany.
Manufacturer
Lilly S.A., Avda. de la Industria 30, 28108 Alcobendas, Madrid, Spain.
Date of Last Revision of this Prospectus:
Detailed information on this medicinal product is available on the European Medicines Agency website http://www.ema.europa.eu/.
INSTRUCTIONS FOR HEALTHCARE PROFESSIONALS
INSTRUCTIONS FOR RECONSTITUTION AND ADMINISTRATION
ZYPADHERA Olanzapine Powder and Solvent for Prolonged-Release Injectable Suspension
FOR INTRAMUSCULAR INJECTION INTO THE GLUTEAL REGION ONLY.
DO NOT ADMINISTER INTRAVENOUSLY OR SUBCUTANEOUSLY.
Reconstitution
STEP 1: Preparation of Materials
The container includes:
- Vial of ZYPADHERA powder for prolonged-release injectable suspension
- Vial of solvent for ZYPADHERA
- A hypodermic syringe and safety needle (hypodermic device)
- A 19-gauge, 38 mm hypodermic safety needle
- Two 19-gauge, 50 mm hypodermic safety needles
- Prospectus
- Reconstitution and Administration Instruction Card (this document)
- Safety Information and Instructions for Use of the Hypodermic Device
|
It is recommended to use gloves as ZYPADHERA may cause skin irritation.
Reconstitute ZYPADHERA powder for prolonged-release injectable suspension exclusively with the solvent supplied in the container using standard aseptic techniques for the reconstitution of parenteral products.
STEP 2: Determination of Solvent Volume for Reconstitution
This table indicates the amount of solvent needed to reconstitute ZYPADHERA powder for prolonged-release injectable suspension.
Concentration of the ZYPADHERA vial (mg) | Volume of solvent to add (ml) |
210 | 1.3 |
300 | 1.8 |
405 | 2.3 |
It is essential to note that the vial contains more solvent than necessary to reconstitute the product.
STEP 3: Reconstitution of ZYPADHERA
- Gently tap the vial to loosen the powder.
- Open the hypodermic syringe and pre-packaged safety needle with the needle protection device. Open the plastic bag and remove the device. Attach the syringe (if not already attached) to the Luer connector of the device with a simple twist. Firmly place the needle onto the device by pushing and twisting it clockwise. Then, directly remove the needle cap. If these instructions are not followed, a needlestick injury may occur.
- Withdraw the predetermined volume of solvent (Step 2) into the syringe.
- Inject the required volume of solvent into the powder vial.
- Withdraw the air to equalize the pressure in the vial.
- Withdraw the needle, with the vial upright to prevent loss of solvent.
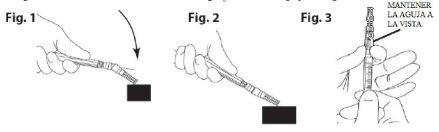
- Put the needle safety device in place. Place the needle into its sheath using a one-handed technique. Perform this maneuver with one hand, making a gentle pressure of the sheath against a flat surface. BY PRESSING ON THE SHEATH (Fig. 1), THE NEEDLE IS SECURELY ATTACHED TO IT (Fig. 2)
- Visually confirm that the needle is completely attached to its protective sheath. Remove the device with the needle attached to the syringe when required through a specific medical procedure. Remove it by holding the Luer connector of the needle protection device with the thumb and index finger, and keeping the other three fingers away from the device where the needle tip is located (Fig. 3).
|
- Vigorously tap the vial against a hard surface several times until no visible powder remains. Protect the surface to cushion the impact. (See Figure A)
|
Figure A: Vigorously tapping to mix
- Visually inspect the vial to identify any clumping of the powder. The powder that is not in suspension has the appearance of pale yellow, dry clumps adhering to the vial. It may be necessary to continue tapping if clumps remain. (See Figure B)
|
Unsuspended: visible clumps Suspended: no clumps
Figure B: Checking for unsuspended powder and tapping if necessary.
- Vigorously shake the vial until the suspension has a uniform appearance with homogeneous color and texture. The suspended product will appear yellow and opaque. (See Figure C)
|
Figure C: Vigorously shaking the vial
If foam forms, let the vial stand until the foam dissipates. If the product is not used immediately, it must be vigorously shaken to achieve resuspension. Reconstituted ZYPADHERA remains stable in the vial for up to 24 hours.
Administration
STEP 1: Inject ZYPADHERA
This table confirms the final volume of ZYPADHERA suspension to be injected. The concentration of the suspension is 150 mg/ml of olanzapine.
Dose (mg) | Final volume to inject (ml) |
150 | 1.0 |
210 | 1.4 |
300 | 2.0 |
405 | 2.7 |
- Determine which needle to use for administering the injection to the patient. For obese patients, 50 mm needles are recommended for injection:
- If the 50 mm needle is used for injection, attach the 38 mm safety needle to the syringe to withdraw the required volume of suspension.
- If the 38 mm needle is used for injection, attach the 50 mm safety needle to withdraw the required volume of suspension.
- Slowly withdraw the desired amount. A small amount of product will remain in the vial.
- Put the needle safety device in place and remove the needle from the syringe.
- Put the safety needle, selecting either the 50 mm or 38 mm needle, onto the syringe before injection. Once the suspension is withdrawn from the vial and transferred to the syringe, it must be injected immediately.
- Select and prepare the injection site in the gluteal region. DO NOT INJECT INTRAVENOUSLY OR SUBCUTANEOUSLY.
- After inserting the needle, aspirate for a few seconds to confirm that no blood is drawn into the syringe. If blood is aspirated into the syringe, discard the syringe and prepare a new suspension. The injection should be performed with firm and continuous pressure.
DO NOT MASSAGE THE INJECTION SITE.
- Put the needle safety device in place. (Fig. 1 and 2)
- Discard the vials, syringe, used needles, additional needle, and remaining solvent according to appropriate clinical procedures. The vial is for single use.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to ZYPADHERA 405 mg POWDER AND SOLVENT FOR PROLONGED-RELEASE INJECTABLE SUSPENSIONDosage form: TABLET, 10 mgActive substance: olanzapineManufacturer: Neuraxpharm Spain S.L.Prescription requiredDosage form: TABLET, 2.5 mgActive substance: olanzapineManufacturer: Neuraxpharm Spain S.L.Prescription requiredDosage form: TABLET, 5 mgActive substance: olanzapineManufacturer: Neuraxpharm Spain S.L.Prescription required
Online doctors for ZYPADHERA 405 mg POWDER AND SOLVENT FOR PROLONGED-RELEASE INJECTABLE SUSPENSION
Discuss questions about ZYPADHERA 405 mg POWDER AND SOLVENT FOR PROLONGED-RELEASE INJECTABLE SUSPENSION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions