ZUTECTRA 500 IU PRE-FILLED SYRINGE SOLUTION FOR INJECTION

How to use ZUTECTRA 500 IU PRE-FILLED SYRINGE SOLUTION FOR INJECTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Zutectra 500UI Solution for Injection in Pre-filled Syringe
Human Immunoglobulin against Hepatitis B
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack:
- What Zutectra is and what it is used for
- What you need to know before you use Zutectra
- How to use Zutectra
- Possible side effects
- Storage of Zutectra
- Contents of the pack and further information
- How to inject Zutectra by yourself or by the person in charge of your care
1. What Zutectra is and what it is used for
What Zutectra is
Zutectra contains antibodies against the hepatitis B virus, which are the body's defensive substances to protect you from hepatitis B. Hepatitis B is an inflammation of the liver caused by the hepatitis B virus.
What Zutectra is used for
Zutectra is used to prevent re-infection with hepatitis B in adults who have had a liver transplant at least 1 week ago, because they have suffered from liver failure caused by hepatitis B.
2. What you need to know before you use Zutectra
Do not use Zutectra
- if you are allergic to human immunoglobulin or to any of the other ingredients of this medicine (listed in section 6).
An allergic reaction can consist of sudden wheezing, difficulty breathing, rapid heartbeat, swelling of the eyelids, face, lips, throat or tongue, skin rash or itching.
Zutectra is for subcutaneous injection (under the skin) only. Injection into a vein or blood vessel can cause an allergic shock.
Warnings and precautions
Tell your doctor or healthcare professional before treatment
- If you have been told that you have antibodies against immunoglobulins of type IgA in your blood. This is very rare and may cause allergic reactions.
You may be allergic to immunoglobulins(antibodies) without knowing it, even if you have tolerated previous treatments with human immunoglobulins. Especially if you do not have enough IgA immunoglobulins in your blood. Allergic reactions can occur, such as a sudden drop in blood pressure or shock.
You will be closely monitored during the first injection with Zutectra and for a short time afterwards, to make sure you do not suffer an allergic reaction. If you experience an allergic reaction to Zutectra, the injection will be stopped immediately. Tell your doctor or healthcare professional immediately if you notice any reaction during the injection with Zutectra.
If you test positive for the HBs antigen, you will not receive Zutectra, as there is no benefit in administering this medicine to you. Your doctor can explain this to you.
For your own safety, your antibody levels will be regularly monitored.
Possible interference with blood tests
Zutectra may affect the results of certain blood tests (serological tests). Tell your doctor about your treatment with Zutectra before any blood test.
Information on the starting material of Zutectra and the possibility of transmitting infectious agents:
The starting material or what Zutectra is made from is human blood plasma (this is the liquid part of the blood).
When medicines are made from human blood or plasma, certain measures are put in place to prevent the transmission of infections to patients. Among others:
- A careful selection of blood and plasma donors to ensure that those who may be at risk of carrying infections are excluded.
- Testing of each donation and plasma pools for signs of viruses or infections.
Manufacturers of these medicines also include steps in the processing of blood or plasma that can inactivate or remove viruses. Despite these measures, it is not possible to completely exclude the risk of transmitting an infection when administering medicines made from human blood or plasma. This also applies to any unknown or emerging virus, or other types of infections.
The measures taken are considered effective for enveloped viruses such as human immunodeficiency virus (HIV), hepatitis B virus, and hepatitis C virus, and for the non-enveloped hepatitis A virus. The measures taken may be of limited value against non-enveloped viruses such as parvovirus B19 (the cause of erythema infectiosum).
Immunoglobulins like Zutectra have not been associated with hepatitis A or parvovirus B19 infections, possibly because the antibodies against these infections, which the product contains, provide protection.
It is strongly recommended that, whenever Zutectra is used (both in the hospital and in home treatment), a record be kept of the patient's name and the batch number of the medicine, in order to maintain a record of the batches used.
Using Zutectra with other medicines
Tell your doctor or healthcare professional if you are using, have recently used, or might use any other medicines.
Vaccinations
Zutectra may reduce the effectiveness of some vaccines (measles, rubella, mumps, chickenpox) for a period of up to 3 months.
You may need to wait at least 3 months after the last injection of Zutectra before you can receive live attenuated vaccines.
Tell your doctor about your treatment with Zutectra before any vaccination.
Pregnancy, breast-feeding, and fertility
If you are pregnant or breast-feeding, think you may be pregnant, or are planning to have a baby, ask your doctor or healthcare professional for advice before using this medicine.
Driving and using machines
Zutectra has no or negligible influence on your ability to drive or use machines.
3. How to use Zutectra
Zutectra is intended for subcutaneous injection (under the skin). The contents of one syringe are intended for single use only. Do not inject into a blood vessel.
In most cases, your doctor or nurse will give you the injection. However, if your antibody levels are sufficient and you have a fixed dose regimen, you or the person in charge of your care may receive instructions to administer the injection at home (see below).
To keep track of your Zutectra injections, it is strongly recommended to use the treatment diary. Your doctor will explain how to use it.
The dose can be determined individually and adjusted from 500 IU to 1,000 IU (in exceptional cases up to 1,500 IU) weekly or biweekly. The dose will depend on your condition. Your doctor will regularly check your health status and inform you about the amount and frequency with which you should use Zutectra.
Injecting Zutectra by yourself or by the person in charge of your care
You can inject Zutectra yourself without the help of your doctor, if you have received instructions to do so. If you are administering Zutectra yourself, read the instructions in the section “How to inject Zutectra by yourself or by the person in charge of your care” carefully.
Zutectra should be allowed to reach room temperature (approximately between 23°C and 27°C) before use.
If you use more Zutectra than you should
The consequences of an overdose are not known. However, if you have used a higher dose of Zutectra than prescribed, contact your doctor, healthcare professional, or pharmacist immediately for advice.
If you forget to use Zutectra
Do not administer a double dose to make up for forgotten injections. Talk to your doctor about how to administer the doses. Your doctor will inform you about the amount and frequency with which you should use Zutectra.
Make sure you use Zutectra as prescribed and as your doctor has told you, in order to avoid the risk of re-infection with hepatitis B.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Most side effects observed with Zutectra were mild to moderate. In very rare cases, human immunoglobulins can cause a severe allergic reaction.
If you notice any of the following, stop the injection and tell your doctor immediately:
- skin rash,
- itching,
- wheezing,
- difficulty breathing,
- swelling of the eyelids, face, lips, throat or tongue,
- low blood pressure, rapid heartbeat
This could be an allergic reaction or a severe allergic reaction (anaphylactic shock).
If you experience any side effect after the injection, talk to your doctor immediately.
The following side effects have been reported with Zutectra:
Common(may affect up to 1 in 10 people):
- Injection site reactions: pain, urticaria at the injection site, hematoma (a collection of blood in the tissue under the skin), redness of the skin
Uncommon(may affect up to 1 in 100 people):
- Headache (cephalalgia)
- upper abdominal pain (from the chest to the navel)
The following reactions have also been reported on a single occasion:
- fatigue (tiredness)
- high blood pressure (hypertension)
- inflammation of the nose and throat (nasopharyngitis)
- muscle spasms
- allergic reactions (hypersensitivity)
- abnormal heart rhythms (palpitations), heart discomfort
- itching, rash
- mouth and throat pain
Additional symptoms have been reported with other human immunoglobulin preparations:
- chills
- headache
- dizziness
- fever
- vomiting
- mild allergic reactions
- nausea (urgent need to vomit)
- joint pain
- low blood pressure
- mild lower back pain (lumbar region)
- injection site reactions: swelling, pain, redness, hardening of the skin, local heat, itching, bruising, and rash.
Reporting of side effects
If you experience any side effect, talk to your doctor, healthcare professional, or pharmacist, even if it is not listed in this leaflet. You can also report side effects directly through the national reporting system listed in Annex V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Zutectra
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the outer packaging and on the label of the syringe after EXP.
Store and transport refrigerated (between 2°C and 8°C).
Keep the container in the outer packaging to protect it from light.
The solution should be administered immediately after opening the syringe.
Do not use Zutectra if you notice that the solution is cloudy or contains particles.
Disposal of unused medicine and all materials that have come into contact with it should be done in accordance with local regulations. Once you have completed the injection, promptly discard all needles, syringes, and empty glass containers in a puncture-resistant container that has been provided to you.
6. Container Contents and Additional Information
Composition ofZutectra
- The active ingredientis human hepatitis B immunoglobulin 500 UI/ml.
- Zutectra contains 150 mg/ml of human plasma protein, of which at least 96% is immunoglobulin G (IgG). The maximum content of immunoglobulin A (IgA) is 6,000 micrograms/ml.
- The other componentsare glycine and water for injectables.
Appearance of the Product and Container Contents
Zutectra is presented as an injectable solution supplied in pre-filled syringes (500 UI/ml; package with five units in a blister). The color of the solution may vary from colorless to pale yellow or light brown.
A pre-filled syringe of 1 ml of Zutectra contains 500 UI. Zutectra is supplied in a package size containing 5 pre-filled syringes, each in a blister-type package.
Marketing Authorization Holder and Manufacturer
Biotest Pharma GmbH
Landsteinerstrasse 5
D–63303 Dreieich
Germany
Tel.: + 49 6103 801–0
Fax: + 49 6103 801–150
e-mail: [email protected]
You can request more information about this medication by contacting the local representative of the marketing authorization holder:
Belgium/Belgique/Belgien, Netherlands Infarama BVBA Stationsstraat 27 B-3570 Alken Tél/Tel: +32 11 31 26 16 | Ireland Aquilant Pharmaceuticals 21 Fonthill Business Park Fonthill Road Clondalkin IRL-Dublin 22 Tel: + 353 1 404 8344 |
Bulgaria Pharmaserv Ltd. 1700 Sofia 11, Tzar Boris III Blvd., fl. 6 T: +359 2 427 49 58 | Italy Grifols Italia S.p.A. Via Torino, 15 I-56010 Vicopisano - Pisa Tel: +39 050 8755111 |
Czech Republic, Slovak Republic Reg-Pharm spol.s.r.o. Fialková 45 CZ-10600 Praha 10 Tel: + 420 2 7265 4004 | Cyprus ΑΚΗΣ ΠΑΝΑΓΙΩΤΟΥ & ΥΙΟΣ ΛΤΔ Γ. ΚΡΑΝΙΔΙΩΤΗ Τ. Θ. 22578 1522 ΛΕΥΚΩΣΙΑ Κ Υ Π Ρ Ο Σ Τηλ: + 357 22 611 038 |
Denmark, Norway, Finland, Sweden Grifols Nordic AB Tel: + 46 8 441 89 50 Email: [email protected] | Hungary Biotest Hungaria Kft. Torbágy u. 15/A H-2045 Törökbálint Tel.: + 36 23 511 311 |
Germany, Estonia, Greece, Iceland, Latvia, Lithuania, Luxembourg/Luxemburg, Poland, Romania, United Kingdom (Northern Ireland) Biotest AG Landsteinerstrasse 5 D-63303 Dreieich Tel: + 49 6103 801-0 | Malta Rodel Ltd 55, Ravina Triq ir-Russett MT-Kappara SGN 4432 Tel: + 356 27 386221 |
Spain Grifols Movaco, S.A. Tel.: +34 93 571 02 00 | Austria Biotest Austria GmbH Einsiedlergasse 58 A-1050 Wien Tel: + 43 1 545 15 61-0 |
France Grifols France 24 Rue de Prony F-75017 Paris Tél: +33 (0) 1 53530870 | Portugal Grifols Portugal, Lda. Tel: +351 219 255 200 |
Croatia Medis Adria d.o.o. Buzinska cesta 58 10010 Zagreb - Buzin T: +385 1 2303 446 | Slovenia MEDIS, d.o.o. Brnciceva 1, SI-1231 Ljubljana-Crnuce,Tel: +386 1 589 69 00 |
Date of the Last Revision of this Leaflet:
Detailed information on this medicinal product is available on the European Medicines Agency website: http://www.ema.europa.eu.
- How to inject Zutectra by yourself or by the person in charge of your care
These instructions are intended to explain how to inject Zutectra. Read the instructions carefully and follow them step by step. Your doctor or their assistant will teach you the administration process.
Do not attempt to inject Zutectra until you are sure you understand how to prepare the injectable solution and administer the injection.
General Information:
- Keep the syringes and the unit for the disposal of the syringe out of the reach of children; store reserves under lock and key if possible.
- Try to give yourself the injection at the same time of day. This makes it easier to remember.
- Always double-check the dose.
- The solution must be at room temperature before use.
- Open each syringe only when you are ready for an injection. You must administer the injection immediately after opening the syringe.
- The color of the solution may vary from colorless to pale yellow or light brown. Do not use solutions that are cloudy or contain particles.
- This medication should not be mixed with other medications.
Before the Injection:
- Wash your hands. It is essential to have your hands and the items you use as clean as possible.
- Have everything you need ready before you start. Find a clean place where you can lay out all the objects you will use:
- two alcohol swabs,
- a Zutectra syringe,
- a suitable needle for subcutaneous injection.
Note that the package does not contain alcohol swabs or needles, and you must provide them yourself.
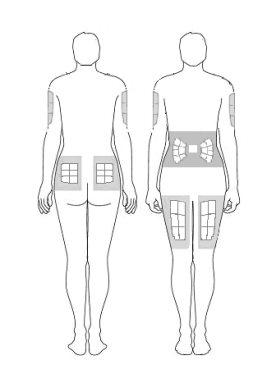
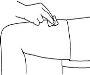
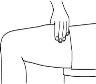
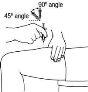
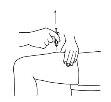
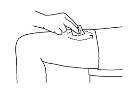
- Before preparing the injection, decide where you will inject. You must inject Zutectra into the fatty layer between the skin and the muscle (about 8 to 12 mm below the skin). The best places for injections are areas of the body where the skin is loose and soft, such as the abdomen, arms, thighs, or buttocks, and away from joints, nerves, or bones.
Important:Do not choose any area where you can feel masses, lumps, firm nodules, pain, or in an area that is discolored, sunken, scarred, or where the skin is torn. Inform your doctor or healthcare professional about these or any other unusual problems you may find. You should change the injection site with each application. If some areas are too difficult for you to reach, you may need someone else to help you with these injections.
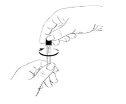
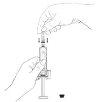
- Prepare the Zutectra syringe:
|
|
|
|
- Expel any air bubbles that may be in the syringe.
|
|
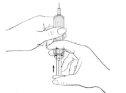
Injection
- Choose the place where you will apply the injection and note it in your diary.
| Abdomen (stomach): Do not use the area within 2.5 cm of the navel. Avoid using the area of the waistline, as friction can irritate the injection site. Avoid surgical scars. This is probably the easiest site for injection if you are doing it yourself. Thighs: Use the middle and outer areas, where you can pinch the tissue. You are likely to have more fatty tissue the closer you are to the hip and the farther you are from the knee. Arms: The back of the arm should be used. It is difficult to pinch the tissue and inject Zutectra by yourself using this site. If you choose to inject yourself in the arm, try pinching the tissue by placing your arm over the back of a chair or against a wall. It is much easier for someone else to use this area if you need help. Buttocks: Use any area where you can pinch the tissue. It is more difficult to administer an injection here yourself. Try standing in front of a mirror to locate the area, or you can ask the person in charge of your care to administer the injection. |
It is essential to change (rotate) the injection sites. This will help keep the skin flexible and help the medication be absorbed evenly. Rotating the sites means starting at one site and using all the other sites before returning to the first site you used. Then start the rotation again. It may be helpful to keep a record of where you last applied the injection to avoid problems.
The following images show an example of administration in the thighs:
|
|
|
|
|
|
|
|
|
|
Disposal of All Used Objects
Once you have given the injection, immediately discard all needles and empty glass containers in a container intended for sharp objects.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to ZUTECTRA 500 IU PRE-FILLED SYRINGE SOLUTION FOR INJECTIONDosage form: INJECTABLE PERFUSION, 50 IU/mLActive substance: hepatitis B immunoglobulinManufacturer: Biotest Pharma GmbhPrescription requiredDosage form: INJECTABLE, 200 IU/mlActive substance: hepatitis B immunoglobulinManufacturer: Instituto Grifols S.A.Prescription requiredDosage form: INJECTABLE, 200 IU/mlActive substance: hepatitis B immunoglobulinManufacturer: Instituto Grifols S.A.Prescription required
Online doctors for ZUTECTRA 500 IU PRE-FILLED SYRINGE SOLUTION FOR INJECTION
Discuss questions about ZUTECTRA 500 IU PRE-FILLED SYRINGE SOLUTION FOR INJECTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions