ZUBSOLV 5.7 mg/1.4 mg SUBLINGUAL TABLETS

How to use ZUBSOLV 5.7 mg/1.4 mg SUBLINGUAL TABLETS
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Zubsolv 0.7 mg / 0.18 mg sublingual tablets
Zubsolv 1.4 mg / 0.36 mg sublingual tablets
Zubsolv 2.9 mg / 0.71 mg sublingual tablets
Zubsolv 5.7 mg / 1.4 mg sublingual tablets
Zubsolv 8.6 mg / 2.1 mg sublingual tablets
Zubsolv 11.4 mg / 2.9 mg sublingual tablets
buprenorphine/naloxone
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Zubsolv and what is it used for
- What you need to know before you take Zubsolv
- How to take Zubsolv
- Possible side effects
- Storing Zubsolv
- Contents of the pack and other information
1. What is Zubsolv and what is it used for
Zubsolv contains the active substances buprenorphine and naloxone. Zubsolv is used to treat opioid dependence (narcotics), such as heroin or morphine, in drug addicts who have agreed to receive treatment for their addiction. Zubsolv is used in adults and adolescents over 15 years of age, who are also receiving medical, social, and psychological support.
How does Zubsolv work?
The tablet contains buprenorphine which is responsible for treating opioid dependence (narcotics). It also contains naloxone which is used to make it difficult to misuse the medicine by intravenous route.
2. What you need to know before you take Zubsolv
Do not take Zubsolv :
- if you are allergic to buprenorphine, naloxone or any of the other ingredients of this medicine (listed in section 6)
- if you have severe respiratory problems
- if you have severe liver problems
- if you have alcohol intoxication or have tremors, sweating, anxiety, confusion or hallucinations caused by alcohol
- if you are taking naltrexone or nalmefene for the treatment of alcohol or opioid dependence
Warnings and precautions
- Misuse, abuse and diversion
Severe infections with potentially fatal outcome may occur if Zubsolv is misused, administered intravenously.
This medicine may be a target for people who abuse prescription medicines, and must be kept in a safe place to protect it from theft (see section 5). Do not give this medicine to anyone else. It may cause them to die or have other harm.
- Respiratory problems(see also “Do not take Zubsolv” above)
Some people have died from respiratory failure (inability to breathe) because they have misused this medicine or taken it in combination with other central nervous system depressants, such as alcohol, benzodiazepines (tranquilizers) or other opioids.
The medicine should be used with caution in patients with pre-existing respiratory problems
This medicine may cause severe, potentially life-threatening respiratory depression in children and in people who are not dependent on opioids if they ingest it accidentally or intentionally.
- Drowsiness
This medicine may cause drowsiness, especially when taken with alcohol or other central nervous system depressants (such as tranquilizers, sedatives or hypnotics).
- Dependence
This medicine may cause dependence.
- Liver damage
Liver damage has been reported after taking buprenorphine/naloxone, especially when the medicine is misused. This may also be due to viral infections (chronic hepatitis C), alcohol abuse, anorexia or the use of other medicines that can damage the liver (see section 4). Your doctor may perform blood tests regularly to monitor your liver.
Tell your doctor if you have liver problems before starting treatment with Zubsolv.
- Withdrawal symptoms
This medicine may cause withdrawal symptoms if you take it less than 6 hours after taking a short-acting opioid (e.g. morphine, heroin) or less than 24 hours after taking a long-acting opioid, such as methadone.
Zubsolv may also cause withdrawal symptoms if you stop taking it abruptly.
- Blood pressure
This medicine may cause a sudden drop in your blood pressure, which would cause a feeling of dizziness if you get up too quickly when sitting or lying down.
- Children and adolescents
You may be under closer supervision by your doctor if you are under 18 years of age.
People under 15 years of age should not take this medicine.
- Diagnosis of unrelated medical conditions
This medicine may mask symptoms of pain that could help in the diagnosis of some diseases. Do not forget to tell your doctor if you are taking this medicine.
Consult your doctor before starting treatment with Zubsolvif:
- you have depression or other illnesses that are treated with antidepressants.
Taking these medicines with Zubsolv may cause serotonin syndrome, a potentially life-threatening disease (see “Taking Zubsolv with other medicines”)
- you have kidney problems
- you have recently suffered a head injury or a brain disease
- you have low blood pressure, enlarged prostate, or difficulty urinating due to a narrowing of the urethra
- you have little activity of the thyroid gland, which can cause fatigue or weight gain
- your adrenal gland does not work well (e.g. Addison's disease)
- you have problems in the bile ducts (e.g. gallbladder, common bile duct)
- you are an elderly person
- you are weakened
Taking Zubsolv with other medicines
Tell your doctor if you are using, have recently used or might use any other medicines.
Some medicines may increase the adverse effects of Zubsolv and in some cases may cause very serious reactions. Do not take other medicines while taking Zubsolv without talking to your doctor first, especially:
- antidepressants such as moclobemide, tranilcipromine, citalopram, escitalopram, fluoxetine, fluvoxamine, paroxetine, sertraline, duloxetine, venlafaxine, amitriptyline, doxepin or trimipramine. These medicines may interact with Zubsolv and you may experience symptoms such as involuntary muscle contractions, including the muscles that control eye movement, agitation, hallucinations, coma, excessive sweating, tremors, exaggerated reflexes, increased muscle tone, body temperature above 38 °C. Contact your doctor if you experience these symptoms.
- Naltrexone and nalmefene(medicines used to treat addictive disorders) as they may prevent the therapeutic effects of Zubsolv. They should not be taken at the same time as treatment with Zubsolv, as you may experience a sudden onset of intense and prolonged withdrawal.
- Benzodiazepines(used to treat anxiety or sleep disorders) such as diazepam, temazepam, alprazolam. Your doctor will indicate the correct dose for you. Taking incorrect doses of benzodiazepines can cause death from respiratory failure (inability to breathe).
- Other medicines that may make you feel drowsyused to treat diseases such as anxiety, insomnia, seizures or epileptic fits, pain and other mental disorders. This type of medicine reduces your alertness, making it dangerous for you to drive and use machines. They can also cause central nervous system depression, which is very serious. The following is a list of examples of this type of medicine:
- other medicines that contain opioids such as methadone, certain painkillers and cough medicines
- certain antidepressants (used to treat depression) such as isocarboxazid, phenelzine, selegiline, tranilcipromine, valproate and monoamine oxidase inhibitors (MAOIs) as they may increase the effects of this medicine
- antihistamines that cause drowsiness (used to treat allergic reactions) such as diphenhydramine and chlorphenamine
- barbiturates (used to induce sleep or sedation) such as phenobarbital, secobarbital
- sedatives (used to induce sleep or sedation) such as chloral hydrate
- clonidine (used to treat high blood pressure) and related medicines as they may prolong the effects of this medicine
- antiretrovirals (used to treat HIV) such as ritonavir, nelfinavir, indinavir, as they may increase the effects of this medicine
- certain antifungal medicines (used to treat fungal infections) such as ketoconazole, itraconazole and certain antibiotics, as they may prolong the effects of this medicine
- some medicines may decrease the effect of Zubsolv. These include medicines used to treat epilepsy (such as carbamazepine and phenytoin) and medicines used to treat tuberculosis (rifampicin)
Using Zubsolv with food, drinks and alcohol
Alcohol may increase drowsiness and may increase the risk of respiratory failure if taken with Zubsolv. Do not take Zubsolv with alcohol.Do not swallow or ingest food or drink until the tablet has completely dissolved.
Pregnancy and breast-feeding
The risks of using Zubsolv in pregnant women are not known. Tell your doctor if you are pregnant or plan to become pregnant. Your doctor will decide if you should continue treatment with an alternative medicine.
When taken during pregnancy, especially towards the end of pregnancy, medicines like Zubsolv may cause withdrawal symptoms, including respiratory problems, in your newborn baby. This may appear several days after birth.
Do not breast-feed while taking this medicine, as Zubsolv passes into breast milk.
Ask your doctor or pharmacist before taking any medicine.
Driving and using machines
Zubsolv may cause drowsiness, dizziness or altered thinking. This may occur more frequently in the first few weeks of treatment, when the dose is being adjusted, but it may also occur if you drink alcohol or take other sedative medicines at the same time as taking Zubsolv. Do not drive, use tools or machines, or engage in hazardous activities, until you know how this medicine affects you.
Zubsolv contains sodium
This medicine contains less than 1 mmol of sodium (23 mg) per dose; this is, essentially “sodium-free”.
3. How to take Zubsolv
Your treatment is prescribed and supervised by doctors with experience in the treatment of drug addiction.
Your doctor will determine the best dose for you. During treatment, the doctor may adjust the dose, based on your response.
Follow the instructions for administration of this medicine exactly as indicated by your doctor or pharmacist. If you are in doubt, consult your doctor or pharmacist.
Starting treatment
The recommended starting dose in adults and adolescents over 15 years of age is:
- one Zubsolv 1.4 mg / 0.36 mg tablet per day, or
- one Zubsolv 2.9 mg / 0.71 mg tablet per day
An additional Zubsolv 1.4 mg / 0.36 mg or 2.9 mg / 0.71 mg tablet may be administered on day 1, depending on your needs.
Other concentrations are available for use by your doctor, who will decide what the best treatment is for you. This may involve taking a combination of different concentrations, but your daily dose should not exceed 17.2 mg of buprenorphine.
Clear signs of withdrawal must be evident before taking your first dose of Zubsolv. Your doctor's assessment of whether you are ready for treatment will guide the timing of your first dose of Zubsolv.
- Starting treatment with Zubsolv if you are dependent on heroin:
If you are dependent on heroin or a short-acting opioid, your first dose of Zubsolv should be taken when withdrawal symptoms appear, but not less than 6 hours after your last opioid use
- Starting treatment with Zubsolv if you are dependent on methadone:
If you have been taking methadone or a long-acting opioid, it is recommended to reduce the methadone dose to below 30 mg/day before starting treatment with Zubsolv. The first dose of Zubsolv should be taken when withdrawal symptoms appear, but not less than 24 hours after you last took methadone
Taking Zubsolv
- Take the dose once a day or as indicated by your doctor.
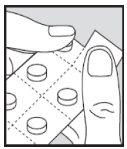
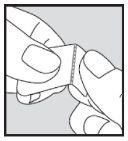
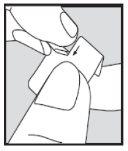
- Remove the tablet as described below. Open the blister immediately before taking the dose. Do not open it in advance, as the tablet is sensitive to moisture.
- Place the tablets under your tongue.
- Keep the tablets under your tongue until they have completely dissolved.
- Do not chew or swallow the tablets, as the medicine will not work and withdrawal symptoms may appear.
- Do not consume food or drink until the tablet has completely dissolved. Although you may notice that most of the tablet disintegrates in 40 seconds, the tablet may take between 5 and 10 minutes to completely disappear from your mouth.
How to remove the tablet from the blister
|
|
|
|
|
|
|
|
Dose adjustment and maintenance treatment
Your doctor may increase the dose of Zubsolv you are taking, based on your needs. If you think the effect of Zubsolv is too strong or too weak, talk to your doctor or pharmacist. The maximum daily dose is 17.2 mg.
After a period of successful treatment, you may agree with your doctor to gradually reduce the dose to a lower maintenance dose.
Stopping treatment
Do not change your treatment in any way or stop treatment without your doctor's authorization.
Depending on your situation, the dose of Zubsolv may continue to be reduced under close medical supervision, until it is discontinued.
If you take more Zubsolv than you should
If you or someone else takes too much of this medicine, you should go or be taken immediately to an emergency center or hospital for treatment, as an overdose of Zubsolv can cause serious, potentially life-threatening breathing problems.
Symptoms of overdose may include slower and weaker breathing than normal, more drowsiness than usual, decreased pupil size, low blood pressure, nausea, vomiting and/or difficulty speaking.
If you forget to take Zubsolv
Tell your doctor as soon as possible if you forget to take a dose.
If you stop taking Zubsolv
Do not change your treatment in any way or stop it without your doctor's authorization. Stopping treatment abruptly can cause withdrawal symptoms.
If you have any other questions about the use of this medicine, ask your doctor or pharmacist.
4. Possible Adverse Effects
Like all medicines, this medicine can cause adverse effects, although not all people suffer from them.
Tell your doctor immediately or seek urgent medical attentionif you experience serious adverse effects, such as:
- swelling of the face, lips, tongue, or throat, which may cause difficulty swallowing or breathing, severe hives/rash. These could be signs of a life-threatening allergic reaction
- drowsiness and lack of coordination, blurred vision, difficulty speaking, inability to think clearly or well, or breathing much slower than normal for you
- severe fatigue, itching with yellowing of the skin or eyes. These may be symptoms of liver damage
- seeing or hearing things that do not exist (hallucinations)
Other Adverse Effects
Very Common Adverse Effects(may affect more than 1 in 10 people):
- insomnia (difficulty sleeping)
- headache
- constipation, nausea
- excessive sweating
- withdrawal syndrome
Common Adverse Effects(may affect up to 1 in 10 people):
- flu-like symptoms, infection, sore throat, and difficulty swallowing, nasal discharge
- anxiety, depression, decreased sexual desire, nervousness, abnormal thoughts
- migraines, dizziness, fainting, increased muscle tension, tingling, drowsiness
- increased tearing (tearful eyes) or other lacrimal disorders
- increased blood pressure, flushing
- increased coughing
- abdominal pain, stomach pain, or other stomach discomfort, diarrhea, flatulence, vomiting
- rash, itching, hives
- back pain, joint pain, muscle pain, leg cramps (muscle spasms)
- urinary disorders
- difficulty getting or maintaining an erection
- weakness, chest pain, chills, fever, general feeling of discomfort, pain, swelling (hands and feet)
- altered liver function, weight loss
- accidental injuries caused by loss of alertness or coordination
Uncommon Adverse Effects(may affect up to 1 in 100 people):
- abnormal blood tests, inflamed lymph nodes
- abnormal dreams, agitation, loss of interest, depersonalization (feeling not like oneself), drug dependence, exaggerated sense of well-being, feelings of hostility
- amnesia (memory disorders), convulsions (seizures), speech disorders, tremors
- eye inflammation or infection, small pupil
- rapid or slow heartbeat, myocardial infarction (heart attack), palpitations, chest tightness
- low blood pressure
- asthma, shortness of breath, yawning
- mouth pain and sores, tongue discoloration
- acne, hair loss, dry or flaky skin, skin nodules
- joint inflammation
- protein in the urine, urinary tract infection, difficulty urinating, painful or difficult urination, blood in the urine, kidney stones
- menstrual or vaginal problems, abnormal ejaculation
- sensitivity to heat or cold
- heat stroke
- excessive muscle activity
- loss of appetite
Frequency Not Known(cannot be estimated from available data):
- slow or difficult breathing
- liver damage with or without jaundice
- hallucinations
- swelling of the face and throat or potentially life-threatening allergic reactions, decrease in blood pressure when changing from a sitting or lying position to standing
- sudden withdrawal syndrome, caused by taking the medicine too soon after illicit opioid use
- withdrawal syndrome in newborn babies
Misuse of this medicine by injection can cause withdrawal symptoms, infections, other skin reactions, and potentially serious liver problems (see section 2, Warnings and Precautions).
Reporting Adverse Effects
If you experience any adverse effects, consult your doctor or pharmacist, even if they are possible adverse effects not listed in this leaflet. You can also report them directly through the Spanish Pharmacovigilance System for Human Use Medicines www.notificaRAM.es. By reporting adverse effects, you can contribute to providing more information on the safety of this medicine.
5. Storage of Zubsolv
Keep this medicine out of sight and reach of children.
Do not use this medicine after the expiration date shown on the box and blister pack after CAD. The expiration date is the last day of the month indicated.
Store in the original package below 25 °C to protect it from moisture.
Zubsolv may be a target for people who misuse prescription medicines. Keep this medicine in a safe place to prevent theft.
Store the blister pack safely.
Never open the blister pack before its time.
Never take this medicine in front of children.
In case of accidental ingestion or suspected ingestion, contact an emergency unit immediately.
Medicines should not be disposed of through wastewater or household waste. Ask your pharmacist how to dispose of the packaging and medicines you no longer need. This will help protect the environment.
6. Package Contents and Additional Information
Zubsolv Composition
The active ingredients are buprenorphine and naloxone.
Each 0.7 mg / 0.18 mg sublingual tablet contains 0.7 mg of buprenorphine (as hydrochloride) and 0.18 mg of naloxone (as hydrochloride dihydrate).
Each 1.4 mg / 0.36 mg sublingual tablet contains 1.4 mg of buprenorphine (as hydrochloride) and 0.36 mg of naloxone (as hydrochloride dihydrate).
Each 2.9 mg / 0.71 mg sublingual tablet contains 2.9 mg of buprenorphine (as hydrochloride) and 0.71 mg of naloxone (as hydrochloride dihydrate).
Each 5.7 mg / 1.4 mg sublingual tablet contains 5.7 mg of buprenorphine (as hydrochloride) and 1.4 mg of naloxone (as hydrochloride dihydrate).
Each 8.6 mg / 2.1 mg sublingual tablet contains 8.6 mg of buprenorphine (as hydrochloride) and 2.1 mg of naloxone (as hydrochloride dihydrate).
Each 11.4 mg / 2.9 mg sublingual tablet contains 11.4 mg of buprenorphine (as hydrochloride) and 2.9 mg of naloxone (as hydrochloride dihydrate).
The other ingredients are mannitol, anhydrous citric acid, sodium citrate, microcrystalline cellulose, sodium croscarmellose, sucralose, levomenthol, colloidal anhydrous silica, and sodium stearyl fumarate.
Zubsolv Appearance and Package Contents
Zubsolv sublingual tablets are available in six different doses, differentiated by shape and engraved inscription:
Zubsolv Tablet Concentration (buprenorphine/naloxone) | Zubsolv Tablet Description | Zubsolv Tablet Engraving | Appearance |
0.7 mg / 0.18 mg | White, oval tablet, 6.8 mm long and 4.0 mm wide | “.7” on one side |
|
1.4 mg / 0.36 mg | White, triangular tablet, 7.2 mm high and 6.9 mm wide | “1.4” on one side |
|
2.9 mg / 0.71 mg | White, D-shaped tablet, 7.3 mm high and 5.65 mm wide | “2.9” on one side |
|
5.7 mg / 1.4 mg | White, round tablet, 7 mm in diameter | “5.7” on one side |
|
8.6 mg / 2.1 mg | White, rhomboid tablet, 9.5 mm long and 8.2 mm wide | “8.6” on one side |
|
11.4 mg / 2.9 mg | White, capsule-shaped tablet, 10.3 mm long and 8.2 mm wide | “11.4” on one side |
|
All tablets will be available in packs of 7, 28, and 30 tablets, in aluminum blisters.
Only some doses and package sizes may be marketed.
Marketing Authorization Holder
Orexo AB
Box 303
751 05 Uppsala
Sweden
Manufacturer
Orexo AB
Virdings allé 32 A Uppsala 751 05
Sweden
Date of Last Revision of this Leaflet:
Detailed information on this medicine is available on the European Medicines Agency website: http://www.ema.europa.eu/.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to ZUBSOLV 5.7 mg/1.4 mg SUBLINGUAL TABLETSDosage form: SUBLINGUAL TABLET, 2 mg/0.5 mgActive substance: buprenorphine, combinationsManufacturer: Aurovitas Spain, S.A.U.Prescription requiredDosage form: SUBLINGUAL TABLET, 8 mg/2 mgActive substance: buprenorphine, combinationsManufacturer: Aurovitas Spain, S.A.U.Prescription requiredDosage form: SUBLINGUAL TABLET, 12 mg/3 mgActive substance: buprenorphine, combinationsManufacturer: Indivior Europe LimitedPrescription required
Online doctors for ZUBSOLV 5.7 mg/1.4 mg SUBLINGUAL TABLETS
Discuss questions about ZUBSOLV 5.7 mg/1.4 mg SUBLINGUAL TABLETS, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions