
ZIMBUS BREEZHALER 114/46/136 micrograms INHALATION POWDER (HARD CAPSULE)

How to use ZIMBUS BREEZHALER 114/46/136 micrograms INHALATION POWDER (HARD CAPSULE)
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Zimbus Breezhaler 114micrograms/46micrograms/136microgramspowder for inhalation (hard capsule)
indacaterol/glycopyrronium/mometasone furoate
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again. If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What Zimbus Breezhaler is and what it is used for
- What you need to know before you use Zimbus Breezhaler
- How to use Zimbus Breezhaler
- Possible side effects
- Storing Zimbus Breezhaler
- Contents of the pack and further information
Instructions for using the Zimbus Breezhaler inhaler
1. What Zimbus Breezhaler is and what it is used for
What Zimbus Breezhaler is and how it works
Zimbus Breezhaler contains three active substances:
- indacaterol
- glycopyrronium
- mometasone furoate
Indacaterol and glycopyrronium belong to a group of medicines called bronchodilators. They work in different ways to relax the muscles of the small airways in the lungs. This helps to open up the airways and makes it easier to breathe in and out. When used regularly, they help the small airways in the lungs to stay open.
Mometasone furoate belongs to a group of medicines called corticosteroids (or steroids). Corticosteroids reduce the swelling and irritation (inflammation) of the small airways in the lungs and in this way gradually relieve breathing problems. Corticosteroids also help to prevent asthma attacks.
What Zimbus Breezhaler is used for
Zimbus Breezhaler is used as a regular treatment for asthma in adults.
Asthma is a serious, chronic lung disease in which the muscles around the smaller airways tighten (bronchoconstriction) and become inflamed. The symptoms come and go and include difficulty breathing, wheezing, chest tightness, and coughing.
You should use Zimbus Breezhaler every day as directed by your doctor and not just when you have breathing problems or other symptoms of asthma. This will ensure that your asthma is adequately controlled. Do not use this medicine to relieve a sudden attack of shortness of breath or wheezing.
Ask your doctor if you have any questions about how Zimbus Breezhaler works or why you have been prescribed this medicine.
2. What you need to know before you use Zimbus Breezhaler
Follow your doctor's instructions carefully.
Do not use Zimbus Breezhaler
- if you are allergic to indacaterol, glycopyrronium, mometasone furoate, or any of the other ingredients of this medicine (listed in section 6). If you think you may be allergic, consult your doctor.
Warnings and precautions
Talk to your doctor, pharmacist, or nurse beforeusing Zimbus Breezhaler if any of the following apply to you:
- if you have heart problems, including a fast or irregular heartbeat,
- if you have thyroid problems,
- if you have been told you have diabetes or high blood sugar levels,
- if you have seizures or fits,
- if you have severe kidney problems,
- if you have severe liver problems,
- if you have low potassium levels in your blood,
- if you have a condition called narrow-angle glaucoma,
- if you have difficulty urinating,
- if you have pulmonary tuberculosis (TB)or any other long-standing or untreated infection.
During treatment with Zimbus Breezhaler
Stop using this medicine and seek medical help immediatelyif you experience any of the following:
- chest tightness, cough, wheezing, or difficulty breathing immediately after using Zimbus Breezhaler (signs that the medicine unexpectedly tightens the airways, known as paradoxical bronchospasm),
- difficulty breathing or swallowing, swelling of the tongue, lips, or face, skin rash, itching, and hives (signs of an allergic reaction),
- eye pain or discomfort, blurred vision, halos, or colored images around lights (signs of an acute attack of narrow-angle glaucoma).
Children and adolescents
Do not give this medicine to children or adolescents (under 18 years of age) because it has not been studied in this age group.
Other medicines and Zimbus Breezhaler
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicines. In particular, tell your doctor or pharmacist if you are using:
- medicines that lower the amount of potassium in the blood. These include diuretics (which increase urine production and may be used to treat high blood pressure, e.g., hydrochlorothiazide), other bronchodilators such as methylxanthines used for respiratory disorders (e.g., theophylline), or corticosteroids (e.g., prednisolone).
- tricyclic antidepressants or monoamine oxidase inhibitors (medicines used to treat depression).
- any medicine that may be similar to Zimbus Breezhaler (containing similar active substances); as their combined use may increase the risk of side effects.
- beta-blocker medicines that may be used for high blood pressure or other heart problems (such as propranolol), or to treat glaucoma (e.g., timolol).
- ketoconazole or itraconazole (medicines used to treat fungal infections).
- ritonavir, nelfinavir, or cobicistat (medicines used to treat HIV infection).
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctor for advice before using this medicine. Your doctor will decide whether you can use Zimbus Breezhaler.
Driving and using machines
Zimbus Breezhaler is unlikely to affect your ability to drive or use machines.
Zimbus Breezhaler contains lactose
This medicine contains approximately 25 mg of lactose per capsule. If your doctor has told you that you have an intolerance to some sugars, consult them before taking this medicine.
3. How to use Zimbus Breezhaler
Follow the instructions for using this medicine exactly as your doctor or pharmacist has told you. If you are unsure, ask your doctor or pharmacist again.
How much Zimbus Breezhaler to inhale
The usual dose is to inhale the contents of one capsule each day. You only need to inhale the medicine once a day. Do not use more doses than your doctor has prescribed.
You should use Zimbus Breezhaler every day, even if you do not feel unwell due to your asthma.
When to inhale Zimbus Breezhaler
Inhale Zimbus Breezhaler at the same time each day. This will help you control your symptoms throughout the day and night. It will also help you remember to use it.
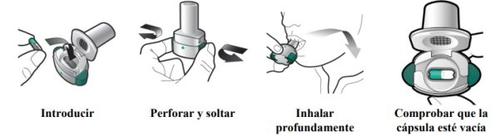
How to inhale Zimbus Breezhaler
- Zimbus Breezhaler is for inhalation use only.
- In this pack, you will find an inhaler and capsules that contain the medicine. The inhaler allows you to inhale the medicine in the capsule. Use the capsules only with the inhaler provided in this pack. The capsules should be kept in the blister pack until you need to use them.
- Peel off the foil to open the blister pack, do not push the capsule through the foil.
- When starting a new pack, use the new inhaler provided in the pack.
- Discard the inhaler from each pack once you have used all the capsules.
- Do not swallow the capsules.
- For more information on how to use the inhaler, please read the instructions at the end of this leaflet.
If your pack contains a sensor for Zimbus Breezhaler
- The sensor and the app are not needed for you to take your medicine. The sensor does not need to be connected to the app when you use your medicine.
- Your doctor will decide if using the sensor and the app is right for you.
- The electronic sensor for Zimbus Breezhaler attaches to the base of the Zimbus Breezhaler inhaler.
- The sensor confirms that you have used the Zimbus Breezhaler inhaler by recording and monitoring the actions and noise made by the capsule when it is turned during inhalation, but it does not monitor whether you have received your dose of medicine.
- The sensor must be used with the Propeller app on your mobile phone or any other suitable device. The sensor connects to the Propeller app via Bluetooth.
- For more information on how to use the sensor for Zimbus Breezhaler and the app, please read the instructions for use provided with the pack containing the sensor and the app.
- Once you have used all the Zimbus Breezhaler capsules from one pack, transfer the sensor to the new inhaler in the next pack of Zimbus Breezhaler.
If your symptoms do not improve
If your asthma does not improve or even gets worse after you have started using Zimbus Breezhaler, talk to your doctor.
If you use more Zimbus Breezhaler than you should
If you accidentally inhale too much of this medicine, contact your doctor or hospital immediately. Medical attention may be needed.
If you forget to use Zimbus Breezhaler
If you miss a dose at the usual time, inhale it as soon as you can on that day. Then, the next day, inhale the next dose at the usual time. Do not inhale two doses on the same day.
If you stop using Zimbus Breezhaler
Do not stop using Zimbus Breezhaler unless your doctor tells you to. Your asthma symptoms may come back if you stop using it.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Some side effects may be serious
Stop using Zimbus Breezhaler and seek medical help immediately if you experience any of the following:
Common:may affect up to 1 in 10 people
- difficulty breathing or swallowing, swelling of the tongue, lips, or face, skin rash, itching, and hives (signs of an allergic reaction).
Other side effects
The following are other side effects. If these side effects begin to be severe, please talk to your doctor, pharmacist, or nurse.
Very common:may affect more than 1 in 10 people
- sore throat
- nasal discharge
- sudden difficulty breathing and feeling of chest tightness with wheezing or coughing
Common:may affect up to 1 in 10 people
- mouth thrush (signs of candidiasis). After you have inhaled your dose, rinse your mouth with water or a mouthwash and spit. This will help prevent thrush.
- frequent urination and pain or burning when urinating (signs of urinary tract infection)
- headache
- fast heartbeat
- cough
- hoarseness
- diarrhea, abdominal cramps, nausea, and vomiting (gastroenteritis)
- muscle, bone, or joint pain (signs of musculoskeletal pain)
- muscle spasms
- fever
Uncommon:may affect up to 1 in 100 people
- dry mouth
- skin rash
- high blood sugar levels
- itching
- difficulty and pain when urinating (signs of dysuria)
- clouding of the lenses in your eyes (signs of cataract)
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storing Zimbus Breezhaler
- Keep this medicine out of the sight and reach of children.
- Do not use this medicine after the expiry date which is stated on the carton and blister after ‘EXP’. The expiry date refers to the last day of that month.
- Do not store above 30°C.
- Store the capsules in the original blister pack to protect them from light and moisture and do not remove them until just before use.
- Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help protect the environment.
- If your pack contains an electronic sensor for Zimbus Breezhaler, please consult the instructions for use provided with the pack containing the sensor for detailed instructions on how to store it and when to discard it.
6. Package Contents and Additional Information
Zimbus Breezhaler Composition
- The active ingredients are indacaterol (as acetate), glycopyrronium (as bromide), and mometasone furoate. Each capsule contains 150 micrograms of indacaterol (as acetate), 63 micrograms of glycopyrronium bromide (equivalent to 50 micrograms of glycopyrronium), and 160 micrograms of mometasone furoate. Each delivered dose (the dose delivered by the inhaler mouthpiece) contains 114 micrograms of indacaterol (as acetate), 58 micrograms of glycopyrronium bromide (equivalent to 46 micrograms of glycopyrronium), and 136 micrograms of mometasone furoate.
- The other ingredients are lactose monohydrate and magnesium stearate (see the section "Zimbus Breezhaler contains lactose" in section 2).
Product Appearance and Package Contents
In this package, you will find an inhaler along with capsules in blisters. Some packages also contain a sensor. The capsules are transparent and contain a white powder. They have the product code "IGM150-50-160" printed in black above two black bars on the body and with the product logo printed in black and surrounded by a black bar on the cap.
The following package sizes are available:
Single pack containing 10 x 1, 30 x 1, or 90 x 1 hard capsules, along with 1 inhaler.
Package containing 30 x 1 hard capsules, along with 1 inhaler and 1 sensor.
Multiple packs containing 15 boxes, each with 10 x 1 capsules along with 1 inhaler.
Only some package sizes may be marketed.
Marketing Authorization Holder
Novartis Europharm Limited
Vista Building
Elm Park, Merrion Road
Dublin 4
Ireland
Manufacturer
Novartis Farmacéutica, S.A.
Gran Via de les Corts Catalanes, 764
08013 Barcelona
Spain
Novartis Pharma GmbH
Roonstraße 25
D-90429 Nuremberg
Germany
Novartis Pharma GmbH
Sophie-Germain-Strasse 10
90443 Nuremberg
Germany
You can request more information about this medicinal product by contacting the local representative of the marketing authorization holder:
België/Belgique/Belgien Novartis Pharma N.V. Tél/Tel: +32 2 246 16 11 | Lietuva SIA Novartis Baltics Lietuvos filialas Tel: +370 5 269 16 50 |
| Luxembourg/Luxemburg Novartis Pharma N.V. Tél/Tel: +32 2 246 16 11 |
Ceská republika Novartis s.r.o. Tel: +420 225 775 111 | Magyarország Novartis Hungária Kft. Tel.: +36 1 457 65 00 |
Danmark Novartis Healthcare A/S Tlf: +45 39 16 84 00 | Malta Novartis Pharma Services Inc. Tel: +356 2122 2872 |
Deutschland Novartis Pharma GmbH Tel: +49 911 273 0 | Nederland Novartis Pharma B.V. Tel: +31 88 04 52 111 |
Eesti SIA Novartis Baltics Eesti filiaal Tel: +372 66 30 810 | Norge Novartis Norge AS Tlf: +47 23 05 20 00 |
Ελλάδα Novartis (Hellas) A.E.B.E. Τηλ: +30 210 281 17 12 | Österreich Novartis Pharma GmbH Tel: +43 1 86 6570 |
España Laboratorios Gebro Pharma, S.A. Tel: +34 93 205 86 86 | Polska Novartis Poland Sp. z o.o. Tel.: +48 22 375 4888 |
France Novartis Pharma S.A.S. Tél: +33 1 55 47 66 00 | Portugal Laboratório Medinfar - Produtos Farmacêuticos, S.A. Tel: +351 21 499 7400 |
Hrvatska Novartis Hrvatska d.o.o. Tel. +385 1 6274 220 | România Novartis Pharma Services Romania SRL Tel: +40 21 31299 01 |
Ireland Novartis Ireland Limited Tel: +353 1 260 12 55 | Slovenija Novartis Pharma Services Inc. Tel: +386 1 300 75 50 |
Ísland Vistor hf. Sími: +354 535 7000 | Slovenská republika Novartis Slovakia s.r.o. Tel: +421 2 5542 5439 |
Italia Novartis Farma S.p.A. Tel: +39 02 96 54 1 | Suomi/Finland Novartis Finland Oy Puh/Tel: +358 (0)10 6133 200 |
Κύπρος Novartis Pharma Services Inc. Τηλ: +357 22 690 690 | Sverige Novartis Sverige AB Tel: +46 8 732 32 00 |
Latvija SIA Novartis Baltics Tel: +371 67 887 070 | United Kingdom (Northern Ireland) Novartis Ireland Limited Tel: +44 1276 698370 |
Date of Last Revision of this Leaflet:
Other Sources of Information
Detailed information on this medicinal product is available on the European Medicines Agency website: http://www.ema.europa.eu.
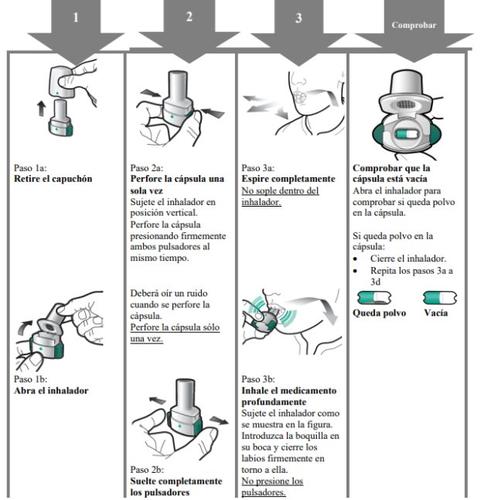
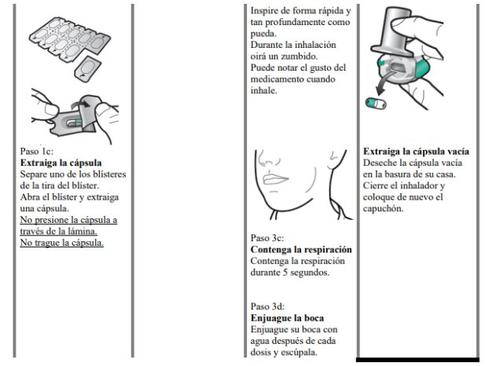
Zimbus Breezhaler Inhaler Instructions for Use
Please read the complete Instructions for Use of the Zimbus Breezhaler inhaler before using it.
- Country of registration
- Average pharmacy price72.68 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to ZIMBUS BREEZHALER 114/46/136 micrograms INHALATION POWDER (HARD CAPSULE)Dosage form: PULMONARY INHALATION, 114 micrograms/46 micrograms/136 microgramsActive substance: indacaterol, glycopyrronium bromide and mometasoneManufacturer: Novartis Europharm LimitedPrescription requiredDosage form: PULMONARY INHALATION, 55 MICROGRAMS/22 MICROGRAMSActive substance: vilanterol and umeclidinium bromideManufacturer: Glaxosmithkline (Ireland) LimitedPrescription requiredDosage form: PULMONARY INHALATION, 340/12 microgramsActive substance: formoterol and aclidinium bromideManufacturer: Covis Pharma Europe B.V.Prescription required
Online doctors for ZIMBUS BREEZHALER 114/46/136 micrograms INHALATION POWDER (HARD CAPSULE)
Discuss questions about ZIMBUS BREEZHALER 114/46/136 micrograms INHALATION POWDER (HARD CAPSULE), including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions
















