ZIEXTENZO 6 mg SOLUTION FOR INJECTION IN PRE-FILLED SYRINGE

How to use ZIEXTENZO 6 mg SOLUTION FOR INJECTION IN PRE-FILLED SYRINGE
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Ziextenzo 6mg solution for injection in pre-filled syringe
pegfilgrastim
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack:
- What is Ziextenzo and what is it used for
- What you need to know before you use Ziextenzo
- How to use Ziextenzo
- Possible side effects
- Storage of Ziextenzo
- Contents of the pack and further information
1. What is Ziextenzo and what is it used for
Ziextenzo contains the active substance pegfilgrastim. Pegfilgrastim is a protein produced by biotechnology in the bacterium E. coli. Pegfilgrastim belongs to a group of proteins called cytokines and is very similar to a natural protein (granulocyte-colony stimulating factor) produced by our body.
Ziextenzo is used to reduce the duration of neutropenia (low white blood cell count) and the incidence of febrile neutropenia (low white blood cell count and fever) that can occur as a result of cytotoxic chemotherapy (medicines that destroy rapidly dividing cells). White blood cells are important cells that help fight infections. These cells are sensitive to the effects of chemotherapy, which can cause their numbers to decrease. If the number of white blood cells becomes too low, there may not be enough to fight off bacteria, which can lead to a higher risk of infection.
Your doctor has prescribed Ziextenzo to stimulate your bone marrow (the part of the bone where blood cells are produced) to produce more white blood cells to help fight infections.
2. What you need to know before you use Ziextenzo
Do not use Ziextenzo
- if you are allergic to pegfilgrastim, filgrastim, or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Talk to your doctor, pharmacist, or nurse before starting Ziextenzo:
- if you experience an allergic reaction that includes weakness, low blood pressure, difficulty breathing, swelling of the face (anaphylaxis), redness and flushing, skin rash, and itching.
- if you experience cough, fever, and difficulty breathing. This may be a sign of acute respiratory distress syndrome (ARDS).
- if you experience any or a combination of the following side effects:
- swelling that may be associated with decreased urine output, difficulty breathing, swelling, and feeling of abdominal fullness and general tiredness.
These may be symptoms of a rare disease called "Capillary Leak Syndrome" and can cause blood to leak from small blood vessels into other parts of your body. See section 4.
- if you have pain in the upper left abdomen or pain in the tip of the shoulder. This may be a sign of a problem with the spleen (splenomegaly).
- if you have recently had a severe lung infection (pneumonia), fluid in the lungs (pulmonary edema), inflammation of the lungs (interstitial lung disease), or an abnormal chest X-ray (pulmonary infiltrate).
- if you are aware of any changes in your blood cell count (e.g., increased white blood cell count or anemia) or a decrease in your platelet count, which can reduce the ability of your blood to clot (thrombocytopenia). Your doctor may want to monitor you more closely.
- if you have sickle cell anemia. Your doctor may monitor your disease more closely.
- if you are a cancer patient, the combined treatment of Ziextenzo with chemotherapy and/or radiotherapy may increase the risk of developing a pre-cancerous blood disorder called myelodysplastic syndrome (MDS) or a blood cancer called acute myeloid leukemia (AML). Symptoms may include tiredness, fever, easy bruising or bleeding.
- if you have sudden signs of allergy, such as rash, itching, or hives on the skin, swelling of the face, lips, tongue, or other parts of the body, shortness of breath, wheezing, or difficulty breathing, these may be signs of a severe allergic reaction.
- if you have symptoms of inflammation of the aorta (the large blood vessel that carries blood from the heart to the rest of the body), this has rarely been reported in patients with cancer and in healthy donors. Symptoms may include fever, abdominal pain, general malaise, back pain, and increased inflammatory markers. Inform your doctor if you experience these symptoms.
Your doctor will regularly perform blood and urine tests as pegfilgrastim may damage the small filters inside the kidneys (glomerulonephritis).
With the use of Ziextenzo, serious skin reactions (Stevens-Johnson syndrome) have been reported. Stop using Ziextenzo and seek medical attention immediately if you notice any of the symptoms described in section 4.
You should discuss with your doctor the risk of developing blood cancer. If you develop or may develop blood cancer, you should not use Ziextenzo, unless your doctor advises you to.
Loss of response to pegfilgrastim
If you experience a loss of response or if the response to treatment with pegfilgrastim cannot be maintained, your doctor will investigate the causes, including whether you have developed antibodies that may neutralize the activity of pegfilgrastim.
Other medicines and Ziextenzo
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicines.
Pregnancy and breastfeeding
Ask your doctor or pharmacist for advice before taking any medicine. Pegfilgrastim has not been used in pregnant women. It is important that you inform your doctor if:
- you are pregnant;
- you think you may be pregnant; or
- you plan to become pregnant.
If you become pregnant during treatment with Ziextenzo, please inform your doctor.
Unless your doctor tells you otherwise, you should stop breastfeeding if you use Ziextenzo.
Driving and using machines
Ziextenzo has no or negligible influence on the ability to drive and use machines.
Ziextenzo contains sorbitol (E 420) and sodium.
This medicine contains 30 mg of sorbitol in each pre-filled syringe, equivalent to 50 mg/ml.
This medicine contains less than 1 mmol of sodium (23 mg) per 6 mg dose; i.e., it is essentially "sodium-free".
3. How to use Ziextenzo
Ziextenzo is indicated in patients aged 18 years and older.
Follow the instructions for administration of Ziextenzo exactly as told by your doctor. If you are unsure, ask your doctor or pharmacist. The usual dose is a single subcutaneous injection of 6 mg (under the skin) using a pre-filled syringe, which should be administered at the end of each chemotherapy cycle, at least 24 hours after your last dose of chemotherapy.
Self-injection of Ziextenzo
Your doctor may consider it more convenient for you to inject Ziextenzo yourself. Your doctor or nurse will teach you how to do this. Do not attempt to self-inject unless you have been trained.
For further instructions on how to self-inject Ziextenzo, read the section at the end of this leaflet.
Do not shake Ziextenzo vigorously as this may affect its activity.
If you use more Ziextenzo than you should
If you use more Ziextenzo than you should, tell your doctor, pharmacist, or nurse.
If you forget to use Ziextenzo
If you are self-injecting and have forgotten to administer your dose of Ziextenzo, contact your doctor to decide when you should inject the next dose.
If you have any further questions on the use of this medicine, ask your doctor, pharmacist, or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Tell your doctor immediately if you experience any or a combination of the following side effects:
- swelling that may be associated with decreased urine output, difficulty breathing, swelling, and feeling of abdominal fullness and general tiredness. These symptoms usually develop very quickly.
These may be symptoms of a rare disease called "Capillary Leak Syndrome" and can cause blood to leak from small blood vessels into other parts of your body and may require urgent medical attention.
Very common side effects(may affect more than 1 in 10 patients):
- bone pain. Your doctor will inform you about what you can take to relieve the pain.
- nausea and headache.
Common side effects(may affect up to 1 in 10 patients):
- injection site pain.
- general pain and pain in the joints and muscles.
- some changes in your blood may occur, which will be detected by regular blood tests. You may have an increased white blood cell count for a short period. You may have a decreased platelet count, which can cause bruising.
Uncommon side effects(may affect up to 1 in 100 patients):
- allergic reactions, including redness and flushing, rash, and skin inflammation with itching.
- severe allergic reactions, including anaphylaxis (weakness, low blood pressure, difficulty breathing, facial swelling).
- enlargement of the spleen.
- rupture of the spleen. Some cases of splenic rupture were fatal. It is important that you contact your doctor immediately if you notice pain in the upper left abdomen or left shoulder tip, as these may be related to a problem with your spleen.
- respiratory problems. If you have a cough, fever, and difficulty breathing, consult your doctor.
- cases of Sweet's syndrome (painful, inflamed, purple-colored lesions on the limbs and sometimes on the face and neck, accompanied by fever) have been reported, but may be related to other factors.
- cutaneous vasculitis (inflammation of the blood vessels in the skin).
- damage to the small filters inside the kidneys (glomerulonephritis).
- redness at the injection site.
- bloody cough (hemoptysis).
- blood disorders (myelodysplastic syndrome [MDS] or acute myeloid leukemia [AML]).
Rare side effects(may affect up to 1 in 1,000 patients):
- inflammation of the aorta (the large blood vessel that carries blood from the heart to the rest of the body), see section 2.
- bleeding in the lungs (pulmonary hemorrhage).
- Stevens-Johnson syndrome, which can appear as red, circular, or concentric patches, often with central blisters, on the trunk, exfoliation, ulcers in the mouth, throat, nose, genitals, and eyes; and may be preceded by fever and flu-like symptoms. Stop using Ziextenzo if you develop these symptoms and contact your doctor or seek medical attention immediately. See section 2.
Reporting of side effects
If you experience any side effects, talk to your doctor, pharmacist, or nurse, even if they are not listed in this leaflet. You can also report side effects directly via the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Ziextenzo
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the carton and on the label of the syringe after EXP. The expiry date is the last day of the month stated.
Store in a refrigerator (2°C to 8°C).
Ziextenzo can be stored at room temperature (below 35°C) for a maximum of 120 hours. Once a syringe has been removed from the refrigerator and has reached room temperature (below 35°C), it must be used within 120 hours or discarded.
Do not freeze. Ziextenzo can be used if accidentally frozen for less than 24 hours.
Keep the container in the outer carton to protect it from light.
Do not use this medicine if you notice that the solution is not entirely clear or contains particles.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Container Contents and Additional Information
Ziextenzo Composition
- The active substance is pegfilgrastim. Each pre-filled syringe contains 6 mg of pegfilgrastim in 0.6 ml of solution.
- The other ingredients are glacial acetic acid, sorbitol (E 420), polysorbate 20, sodium hydroxide, and water for injections. See section 2 "Ziextenzo contains sorbitol (E 420) and sodium".
Product Appearance and Container Contents
Ziextenzo is a clear, colorless to slightly yellowish injectable solution (injection) in a pre-filled syringe (6 mg/0.6 ml).
Each container contains 1 pre-filled glass syringe with a rubber plunger stopper (bromobutyl rubber, latex-free), a plunger rod, a stainless steel needle of 29 gauge, and a needle shield cap (thermoplastic elastomer, latex-free). The syringes are supplied with an automatic needle guard.
Marketing Authorization Holder
Sandoz GmbH
Biochemiestr. 10
6250 Kundl
Austria
Manufacturer
Sandoz GmbH
Biochemiestr. 10
6336 Langkampfen
Austria
Novartis Pharmaceutical Manufacturing GmbH
Biochemiestrasse 10
6336 Langkampfen
Austria
You can request more information about this medicinal product from the local representative of the marketing authorization holder:
Belgium/Belgique/Belgien Sandoz nv/sa Tel: +32 2 722 97 97 | Lithuania Sandoz Pharmaceuticals d.d filialas Tel: +370 5 2636 037 |
Bulgaria Sandoz Pharmaceuticals EAD Tel: +359 2 970 47 47 | Luxembourg/Luxemburg Sandoz nv/sa Tel: +32 2 722 97 97 |
Czech Republic Sandoz s.r.o. Tel: +420 225 775 111 | Hungary Sandoz Hungária Kft. Tel: +36 1 430 2890 |
Denmark/Norway/Iceland/Sweden Sandoz A/S Tel: +45 63 95 10 00 | Malta Sandoz Pharmaceuticals d.d. Tel: +35699644126 |
Germany Hexal AG Tel: +49 8024 908 0 | Netherlands Sandoz B.V. Tel: +31 36 52 41 600 |
Estonia Sandoz d.d. Eesti filiaal Tel: +372 665 2400 | Austria Sandoz GmbH Tel: +43 5338 2000 |
Greece SANDOZ HELLAS ΜΟΝΟΠΡΟΣΩΠΗ Α.Ε. Tel: +30 216 600 5000 | Poland Sandoz Polska Sp. z o.o. Tel: +48 22 209 70 00 |
Spain Sandoz Farmacéutica, S.A. Tel: +34 900 456 856 | Portugal Sandoz Farmacêutica Lda. Tel: +351 21 000 86 00 |
France Sandoz SAS Tel: +33 1 49 64 48 00 | Romania Sandoz Pharmaceuticals SRL Tel: +40 21 407 51 60 |
Croatia Sandoz d.o.o. Tel: +385 1 23 53 111 | Slovenia Sandoz farmacevtska družba d.d. Tel: +386 1 580 29 02 |
Ireland Rowex Ltd. Tel: + 353 27 50077 | Slovakia Sandoz d.d. - organizačná zložka Tel: +421 2 48 20 0600 |
Italy Sandoz S.p.A. Tel: +39 02 96541 | Finland Sandoz A/S Tel: +358 10 6133 400 |
Cyprus Sandoz Pharmaceuticals d.d. Tel: +357 22 69 0690 | United Kingdom (Northern Ireland) Sandoz GmbH Tel: +43 5338 2000 |
Latvia Sandoz d.d. Latvia filiale Tel: +371 67 892 006 |
Date of Last Revision of this Leaflet:.
Other Sources of Information
Detailed information on this medicinal product is available on the European Medicines Agency website: http://www.ema.europa.eu.
The leaflet for this product can be found on the European Medicines Agency website in all EU/EEA languages.
Instructions for Using the Ziextenzo Pre-filled Syringe with Needle Guard
To help avoid possible infections and to ensure you use the medicine correctly, it is important that you follow these instructions.
Read ALL the instructions before injecting yourself. It is important that you do not attempt to self-inject until you have been taught how to do it by a doctor, nurse, or pharmacist. The box contains the pre-filled syringe sealed individually in a plastic blister.
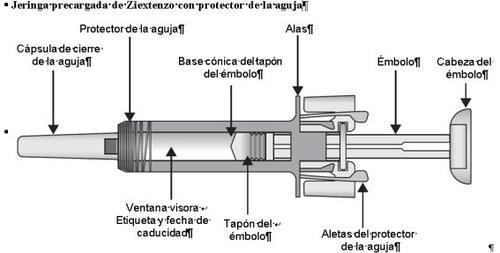
After injecting the medicine, the needle guard is activated to cover the needle. The purpose of the needle guard is to prevent healthcare professionals, caregivers, and patients from accidentally pricking themselves with the needle after injection.
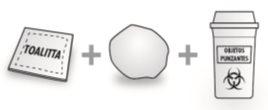
What you will need for injection:
|
|
Important Safety Information
Caution: Keep the pre-filled syringe out of sight and reach of children.
- Do not open the box until you are ready to use the pre-filled syringe.
- Do not use the pre-filled syringe if the blister seal is broken, as it may not be safe to use.
- Do not use the pre-filled syringe if there is liquid in the blister. Do not use the pre-filled syringe if the needle shield is missing or not properly placed. In this case, return the entire product container to the pharmacy.
- Never leave the pre-filled syringe unattended where others may handle it.
- Do not shake the pre-filled syringe.
- Avoid touching the wings of the needle guard before use. If you touch them, the needle guard may activate too early.
- Do not remove the needle shield until the moment of administration.
- The pre-filled syringe cannot be reused. Discard the used syringe immediately after use in a sharps container.
- Do not use if the syringe has been dropped onto a hard surface or dropped after removing the needle cap.
Storage of the Ziextenzo Pre-filled Syringe
- Store the pre-filled syringe in the blister pack in the carton to protect it from light.
- Store in a refrigerator between 2 °C and 8 °C. Do not freeze.
- Before using, remove the pre-filled syringe from the refrigerator and let Ziextenzo reach room temperature (up to a maximum of 35 °C) for approximately 15-30 minutes.
- Do not usethe pre-filled syringe after the expiration date stated on the carton or syringe label. If it has expired, return the entire container to the pharmacy.
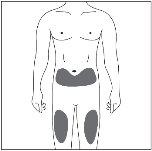
Injection Site
| The injection site is the place on your body where you will use the pre-filled syringe.
|
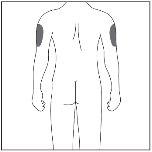
| If the injection is administered by a caregiver, the upper outer arm can also be used. |
Preparing the Ziextenzo Pre-filled Syringe for Use
- Remove the carton containing the pre-filled syringe in the blister pack from the refrigerator and let it unopenedfor approximately 15-30 minutes to reach room temperature.
- When you are ready to use the pre-filled syringe, open the blister pack and wash your hands well with soap and water.
- Clean the injection site with an alcohol swab.
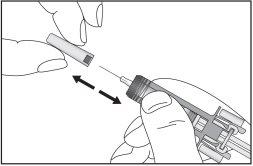
- Remove the pre-filled syringe from the blister pack by holding it in the middle, as shown below. Do not touch the plunger. Do not touch the needle shield.
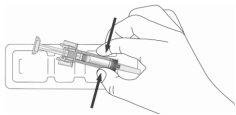
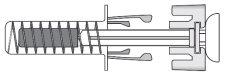
- Check that the transparent plastic needle guard is placed over the glass syringe body. If the transparent needle guard covers the needle shield (as illustrated below), the syringe guard has been activated and this syringe must notbe used; use a new one instead. The figure below shows a ready-to-use syringe.
DEVICE ACTIVATED – DO NOT USE
| In this configuration, the needle guard isACTIVATED – DO NOT USEthe pre-filled syringe |
DEVICE READY FOR USE
| In this configuration, the needle guard isDEACTIVATEDand the pre-filled syringe is ready for use |
- Inspect the pre-filled syringe. The liquid should be clear, colorless to slightly yellowish. A small air bubble may be visible in the liquid, but this is normal. Do not usethe pre-filled syringe if other particles or discoloration are seen.
- Do not usethe syringe if it is broken or activated. Return the Ziextenzo pre-filled syringe and container to the pharmacy.
Using the Ziextenzo Pre-filled Syringe
1
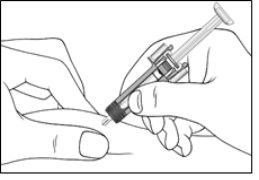
| Gently pull the needle shield straight off. Discard the needle shield. There may be a drop of liquid at the needle tip. This is normal. |
2
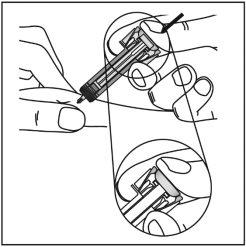
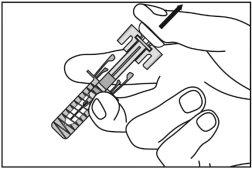
| Gently pinch the skin at the injection site and insert the needle as shown. Push the needle all the way in to ensure that all the medicine can be administered. |
3
| Hold the pre-filled syringe as shown, and slowlypress the plunger all the waydownuntil the plunger head is fully between the wings of the needle guard. Keep the syringe in this position with the plunger fully pressed for 5 seconds. |
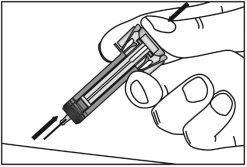
4
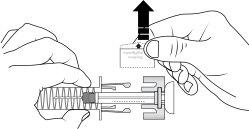
| With the plunger fullypressed, carefully pull the needle out of the injection site at the same angle it was inserted and release the skin. |
5
| Release the plunger slowly and let the needle guard of the syringe automatically cover the exposed needle. There may be a little blood at the injection site. You can press on the injection site with a cotton ball or gauze for 10 seconds. Do not rub the injection site; if necessary, you can cover it with a small adhesive bandage. |
6
| For healthcare professionals only The trade name of the administered product must be properly recorded in the patient's medical history. Remove and store the pre-filled syringe label. Turn the plunger to move the syringe label to a position where you can remove it. |
7
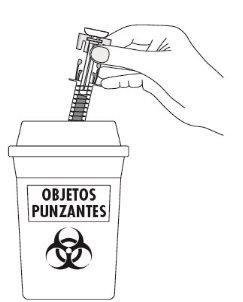
| Disposal Instructions Place the used syringe in a sharps container (a puncture-resistant and closable container). Medicines should not be disposed of via wastewater or household waste. Ask your doctor or pharmacist how to dispose of medicines no longer required. This will help protect the environment. The disposal of unused medicinal products and all materials that have come into contact with them will be carried out in accordance with local regulations. |
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to ZIEXTENZO 6 mg SOLUTION FOR INJECTION IN PRE-FILLED SYRINGEDosage form: INJECTABLE, 6 mgActive substance: pegfilgrastimManufacturer: Biosimilar Collaborations Ireland LimitedPrescription requiredDosage form: INJECTABLE, 6 mg of pegfilgrastim (10mg/ml)Active substance: pegfilgrastimManufacturer: Amgen Europe B.V.Prescription requiredDosage form: INJECTABLE, 6 mgActive substance: pegfilgrastimManufacturer: Amgen Europe B.V.Prescription required
Online doctors for ZIEXTENZO 6 mg SOLUTION FOR INJECTION IN PRE-FILLED SYRINGE
Discuss questions about ZIEXTENZO 6 mg SOLUTION FOR INJECTION IN PRE-FILLED SYRINGE, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions