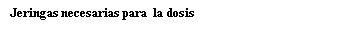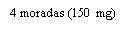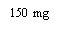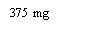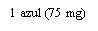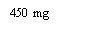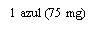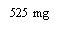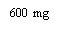XOLAIR 150 mg INJECTABLE SOLUTION

How to use XOLAIR 150 mg INJECTABLE SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Xolair 150mg solution for injection in pre-filled syringe
(pre-filled syringe with fixed 26-gauge needle, purple syringe protector)
omalizumab
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Xolair and what is it used for
- What you need to know before you use Xolair
- How to use Xolair
- Possible side effects
- Storage of Xolair
- Contents of the pack and other information
1. What is Xolair and what is it used for
Xolair contains the active substance omalizumab. Omalizumab is a human protein, similar to the natural proteins produced by the body. It belongs to a class of medicines called monoclonal antibodies.
Xolair is used for the treatment of:
- allergic asthma
- chronic rhinosinusitis (inflammation of the nose and sinuses) with nasal polyps
- chronic spontaneous urticaria (CSU)
Allergic asthma
This medicine is used to prevent asthma from getting worse by controlling symptoms of severe allergic asthma in adults, adolescents, and children (from 6 years of age) who are already taking asthma medicines, but whose symptoms are not adequately controlled with medicines such as high-dose inhaled corticosteroids and inhaled beta-agonists.
Chronic rhinosinusitis with nasal polyps
This medicine is used to treat chronic rhinosinusitis with nasal polyps in adults (from 18 years of age) who are taking intranasal corticosteroids (nasal spray with corticosteroids), but whose symptoms are not well controlled with these medicines. Nasal polyps are small growths in the lining of the nose. Xolair helps to reduce the size of the polyps and improves symptoms including nasal congestion, loss of smell, mucus in the back of the throat, and nasal discharge.
Chronic spontaneous urticaria (CSU)
This medicine is used for the treatment of chronic spontaneous urticaria in adults and adolescents (from 12 years of age) who are already taking antihistamines but whose CSU symptoms are not well controlled by these medicines.
Xolair works by blocking a substance called immunoglobulin E (IgE) that is produced by the body. IgE is involved in a type of inflammation that plays a key role in causing allergic asthma, chronic rhinosinusitis with nasal polyps, and CSU.
2. What you need to know before you use Xolair
Do not use Xolair
- if you are allergic to omalizumab or any of the other ingredients of this medicine (listed in section 6).
If you think you may be allergic to any of the ingredients, tell your doctor, as you should not use Xolair.
Warnings and precautions
Tell your doctor before using Xolair:
- if you have kidney or liver problems,
- if you have a condition where your immune system attacks parts of your body (autoimmune disease),
- if you are traveling to an area where parasitic infections are common, as Xolair may reduce your resistance to these infections,
- if you have had a severe allergic reaction (anaphylaxis) in the past, for example, as a result of taking a medicine, an insect bite, or food,
- if you have had an allergic reaction to latex. The needle cap of the pre-filled syringe may contain dry natural rubber (latex).
Xolair does not treat acute asthma symptoms, such as a sudden asthma attack. Therefore, Xolair should not be used to treat this type of symptom.
Xolair is not intended to prevent or treat other allergic conditions, such as sudden allergic reactions, hyperimmunoglobulin E syndrome (a rare inherited immune disorder), aspergillosis (a fungal disease of the lungs), food allergy, eczema, or hay fever, as Xolair has not been studied in these conditions.
Monitor for signs of allergic reactions and other serious side effects
Xolair may cause serious side effects. You should monitor for signs of these effects while using Xolair. Seek medical attention immediately if you notice any signs that indicate a severe allergic reaction or other serious side effects. These signs are mentioned in "Serious side effects" in section 4.
Before you or someone else injects Xolair, it is essential that you receive training from your doctor on how to recognize the early symptoms of severe allergic reactions and how to act if they occur (see section 3, "How to use Xolair"). Most severe allergic reactions occur during the first three doses of Xolair.
Children and adolescents
Allergic asthma
Xolair is not recommended for children under 6 years of age. Its use in children under 6 years of age has not been studied.
Chronic rhinosinusitis with nasal polyps
Xolair is not recommended for children and adolescents under 18 years of age. Its use in patients under 18 years of age has not been studied.
Chronic spontaneous urticaria (CSU)
Xolair is not recommended for children under 12 years of age. Its use in children under 12 years of age has not been studied.
Other medicines and Xolair
Tell your doctor, pharmacist, or nurse if you are taking, have recently taken, or might take any other medicines.
This is especially important if you are using:
- medicines to treat a parasitic infection, as Xolair may reduce the effect of your medicines,
- inhaled corticosteroids and other medicines for allergic asthma.
Pregnancy and breastfeeding
If you are pregnant, think you may be pregnant, or plan to become pregnant, consult your doctor before using this medicine. Your doctor will discuss with you the potential benefits and risks of using this medicine during pregnancy.
Tell your doctor immediately if you become pregnant while being treated with Xolair.
Xolair may pass into breast milk. If you are breastfeeding or plan to breastfeed, consult your doctor before using this medicine.
Driving and using machines
Xolair is unlikely to affect your ability to drive or use machines.
3. How to use Xolair
Follow exactly the instructions for administration of this medicine given by your doctor. If you are unsure, consult your doctor, pharmacist, or nurse again.
How to use Xolair
Xolair is used as an injection under the skin (known as a subcutaneous injection).
Xolair injection
- You and your doctor will decide if you will inject Xolair yourself. The first three doses will always be injected under the supervision of a healthcare professional (see section 2).
- It is essential that you have received proper training on how to inject the medicine before you do it yourself.
- The caregiver (e.g., parents) can give the Xolair injection after proper training.
For detailed instructions on how to inject Xolair, see "Instructions for use of Xolair in pre-filled syringe" at the end of this leaflet.
Training to recognize severe allergic reactions
It is also essential that you do not inject Xolair yourself until your doctor or nurse has taught you:
- how to recognize the signs and symptoms of severe allergic reactions,
- what to do if symptoms appear.
For more information on the signs and symptoms of severe allergic reactions, see section 4.
How much to use
Allergic asthma and chronic rhinosinusitis with nasal polyps
Your doctor will decide how much Xolair you need and how often you should use it. This depends on your body weight and the results of a blood test done before starting treatment to determine the level of IgE in your blood.
You will need between 1 and 4 injections at the same time. You will need injections every 2 or 4 weeks.
Continue taking your current asthma and/or nasal polyp medicines while using Xolair. Do not stop any asthma and/or nasal polyp medicines without consulting your doctor.
You may not notice an immediate improvement after starting treatment with Xolair. In patients with nasal polyps, the effects have been observed 4 weeks after starting treatment. In patients with asthma, it usually takes between 12 and 16 weeks for the medicine to take full effect.
Chronic spontaneous urticaria (CSU)
You will need two 150 mg injections at the same time every 4 weeks.
Continue taking your current CSU medicines during treatment with Xolair. Do not stop any medicines without consulting your doctor.
Use in children and adolescents
Allergic asthma
Xolair can be used in children and adolescents from 6 years of age who are already taking asthma medicines, but whose asthma symptoms are not well controlled by medicines such as high-dose inhaled corticosteroids and inhaled beta-agonists. Your doctor will tell you how much Xolair your child needs and how often it should be used. This will depend on the child's weight and the results of blood tests done before starting treatment to determine the level of IgE in their blood.
Children (from 6 to 11 years of age) are not expected to inject Xolair themselves. However, if the doctor considers it appropriate, the caregiver can give the injection after proper training.
Chronic rhinosinusitis with nasal polyps
Xolair should not be used in children and adolescents under 18 years of age.
Chronic spontaneous urticaria (CSU)
Xolair can be used in adolescents from 12 years of age who are already taking antihistamines but whose CSU symptoms are not well controlled by these medicines. The dose for adolescents from 12 years of age is the same as for adults.
If you miss a dose of Xolair
If you miss a visit, contact your doctor or hospital as soon as possible to reschedule.
If you miss injecting a dose of Xolair, inject it as soon as you remember. Then, consult your doctor to find out when you should have your next dose.
If you stop using Xolair
Do not stop using Xolair unless your doctor tells you to. Stopping or ending treatment with Xolair may cause your symptoms to come back.
However, if you are being treated for CSU, your doctor may stop Xolair treatment from time to time to assess your symptoms. Follow your doctor's instructions.
If you have any further questions on the use of this medicine, ask your doctor, pharmacist, or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them. The side effects caused by Xolair are usually mild to moderate, but occasionally they can be serious.
Serious side effects:
Seek medical attention immediately if you notice any of the signs of the following side effects:
Rare (may affect up to 1 in 1,000 people)
- Severe allergic reactions (including anaphylaxis). Symptoms may include rash, itching, hives, swelling of the face, lips, tongue, larynx (voice box), trachea, or other parts of the body, rapid heartbeat, dizziness, and lightheadedness, confusion, shortness of breath, wheezing, or difficulty breathing, blue-tinged skin or lips, collapse, and loss of consciousness. If you have a history of severe allergic reactions (anaphylaxis) not related to Xolair, you may be at greater risk of developing a severe allergic reaction after using Xolair.
- Systemic lupus erythematosus (SLE). Symptoms may include muscle pain, joint pain and swelling, rash, fever, weight loss, and fatigue.
Frequency not known (cannot be estimated from the available data)
- Churg-Strauss syndrome or hypereosinophilic syndrome. Symptoms may include one or more of the following: swelling, pain, or rash around blood vessels or lymph nodes, high levels of a specific type of white blood cell (marked eosinophilia), worsening respiratory problems, nasal congestion, heart problems, pain, numbness, or tingling in the arms and legs.
- Low blood platelet count with symptoms such as bleeding or bruising that occurs more easily than normal.
- Serum sickness. Symptoms may include one or more of the following: joint pain with or without swelling or stiffness, rash, fever, swelling of the lymph nodes, muscle pain.
Other side effects include:
Very common (may affect more than 1 in 10 people)
- fever (in children)
Common (may affect up to 1 in 10 people)
- injection site reactions including pain, swelling, itching, and redness
- upper stomach pain
- headache (very common in children)
- upper respiratory tract infections, such as pharyngitis and common cold
- pressure or pain in the cheeks and forehead (sinusitis, sinus headache)
- joint pain (arthralgia)
- feeling dizzy
Uncommon (may affect up to 1 in 100 people)
- feeling sleepy or tired
- tingling or numbness of hands or feet
- fainting, decreased blood pressure when sitting or standing up (postural hypotension), flushing
- sore throat, cough, acute respiratory problems
- feeling sick (nausea), diarrhea, indigestion
- itching, hives, rash, increased sensitivity of the skin to the sun
- weight gain
- flu-like symptoms
- swollen arms
Rare (may affect up to 1 in 1,000 people)
- parasitic infection
Frequency not known (cannot be estimated from the available data)
- muscle pain and joint inflammation
- hair loss
Reporting of side effects
If you experience any side effects, talk to your doctor, pharmacist, or nurse, even if it is possible that they are not listed in this leaflet. You can also report side effects directly via the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Xolair
- Keep this medicine out of the sight and reach of children.
- Do not use this medicine after the expiry date which is stated on the label after EXP. The expiry date is the last day of the month stated.
- Store in the original package to protect from light.
- Store in a refrigerator (2°C to 8°C). Do not freeze.
- Do not use any packaging that is damaged or shows signs of deterioration.
6. Container Contents and Additional Information
Xolair Composition
- The active ingredient is omalizumab. A 1 ml syringe of solution contains 150 mg of omalizumab.
- The other components are arginine hydrochloride, histidine monohydrochloride, histidine, polysorbate 20, and water for injectable preparations.
- The needle shield of the syringe may contain dry rubber (latex).
Product Appearance and Container Contents
Xolair injectable solution is presented as a clear to slightly opalescent, colorless to pale yellowish-brown solution in a pre-filled syringe.
Xolair 150 mg injectable solution in a pre-filled syringe with a fixed 26-gauge needle and a purple syringe shield is available in packs containing 1 pre-filled syringe and in multipacks containing 4 (4 x 1), 6 (6 x 1), or 10 (10 x 1) pre-filled syringes.
Not all pack sizes may be marketed.
Marketing Authorization Holder
Novartis Europharm Limited
Vista Building
Elm Park, Merrion Road
Dublin 4
Ireland
Manufacturer
Novartis Farmacéutica S.A.
Gran Via de les Corts Catalanes, 764
08013 Barcelona
Spain
Novartis Pharma GmbH
Roonstrasse 25
D-90429 Nuremberg
Germany
Novartis Pharma GmbH
Sophie-Germain-Strasse 10
90443 Nürnberg
Germany
You can request more information about this medicinal product from the local representative of the marketing authorization holder:
Belgium/Belgique/Belgien Novartis Pharma N.V. Tel: +32 2 246 16 11 | Lithuania SIA Novartis Baltics Lietuvos filialas Tel: +370 5 269 16 50 |
Novartis Bulgaria EOOD Tel: +359 2 489 98 28 | Luxembourg/Luxemburg Novartis Pharma N.V. Tel: +32 2 246 16 11 |
Czech Republic Novartis s.r.o. Tel: +420 225 775 111 | Hungary Novartis Hungária Kft. Tel: +36 1 457 65 00 |
Denmark Novartis Healthcare A/S Tel: +45 39 16 84 00 | Malta Novartis Pharma Services Inc. Tel: +356 2122 2872 |
Germany Novartis Pharma GmbH Tel: +49 911 273 0 | Netherlands Novartis Pharma B.V. Tel: +31 88 04 52 111 |
Estonia SIA Novartis Baltics Eesti filiaal Tel: +372 66 30 810 | Norway Novartis Norge AS Tel: +47 23 05 20 00 |
Greece Novartis (Hellas) A.E.B.E. Tel: +30 210 281 17 12 | Austria Novartis Pharma GmbH Tel: +43 1 86 6570 |
Spain Novartis Farmacéutica, S.A. Tel: +34 93 306 42 00 | Poland Novartis Poland Sp. z o.o. Tel: +48 22 375 4888 |
France Novartis Pharma S.A.S. Tel: +33 1 55 47 66 00 | Portugal Novartis Farma - Produtos Farmacêuticos, S.A. Tel: +351 21 000 8600 |
Croatia Novartis Hrvatska d.o.o. Tel: +385 1 6274 220 | Romania Novartis Pharma Services Romania SRL Tel: +40 21 31299 01 |
Ireland Novartis Ireland Limited Tel: +353 1 260 12 55 | Slovenia Novartis Pharma Services Inc. Tel: +386 1 300 75 50 |
Iceland Vistor hf. Tel: +354 535 7000 | Slovakia Novartis Slovakia s.r.o. Tel: +421 2 5542 5439 |
Italy Novartis Farma S.p.A. Tel: +39 02 96 54 1 | Finland Novartis Finland Oy Tel: +358 (0)10 6133 200 |
Cyprus Novartis Pharma Services Inc. Tel: +357 22 690 690 | Sweden Novartis Sverige AB Tel: +46 8 732 32 00 |
Latvia SIA Novartis Baltics Tel: +371 67 887 070 |
Date of Last Revision of this Prospectus:
Other Sources of Information
Detailed information on this medicinal product is available on the European Medicines Agency website: http://www.ema.europa.eu
XOLAIR PRE-FILLED SYRINGE INSTRUCTIONS FOR USE
Read ALL the instructions before injecting the medicine. If your doctor decides that you or the person caring for you can administer your Xolair injections at home, you will need to receive training from your doctor, nurse, or pharmacist before you inject the medicine or inject it into others. It is not expected that children (from 6 years to less than 12 years of age) will self-inject Xolair; however, if your doctor considers it appropriate, their caregiver may inject Xolair after receiving proper training. The box contains the Xolair pre-filled syringe(s) individually closed in a plastic tray.
Your Xolair 150 mg Pre-filled Syringe Injectable Solution




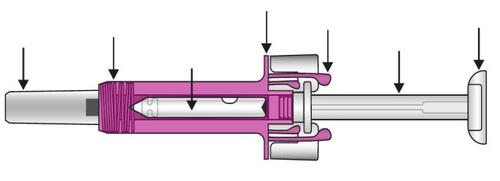
Once the medicine has been injected, the syringe shield will activate to cover the needle. This is designed to protect against accidental needlestick injuries.
What you will need for the injection:
|
|
Important Safety Information
Warning: Keep the syringe out of sight and reach of children.
- The needle cap of the syringe may contain dry rubber (latex) that should not be touched by people sensitive to this substance.
- Do not open the sealed box until you are ready to use this medicine.
- Do not use this medicine if the seal of the box or the plastic tray is broken, as it may not be safe to use.
- Do not use if the syringe has been dropped onto a hard surface or has been dropped after removing the needle cap.
- Never leave the syringe in places where others may touch it.
- Do not shake the syringe.
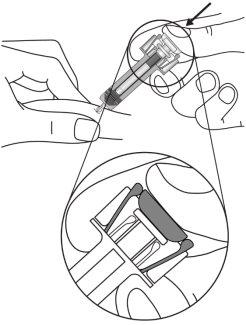
- Be very careful not to touch the activation clips before use. If you do, the needle shield will activate too early.
- Do not remove the needle cap until just before injecting.
- The syringe cannot be reused. Once used, discard the syringe in the sharps disposal container.
Storage of Xolair Pre-filled Syringe Injectable Solution
- Store this medicine sealed in its box to protect it from light. Store in a refrigerator at 2°C to 8°C. DO NOT FREEZE.
- Remember to remove the syringe from the refrigerator to allow it to reach room temperature (25°C) before preparing the injection (this will take approximately 30 minutes). Leave the syringe in the box to protect it from light. The total time the syringe can remain at room temperature (25°C) before use should not exceed 48 hours.
- Do not use the syringe after the expiry date shown on the box or on the syringe label. If it has expired, return the complete package to the pharmacy.
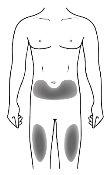
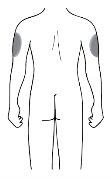
Injection Site
| The injection site is where you will use the syringe
If the person injecting is the caregiver, the upper arm can also be used. |
Preparing Xolair Pre-filled Syringe Injectable Solution for Use
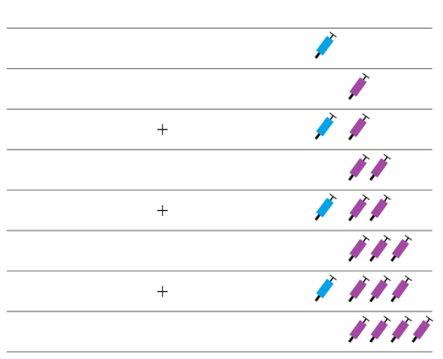
Note: Depending on the dose prescribed by your doctor, you may need to prepare one or more pre-filled syringes and inject the contents of all of them. The following table provides examples of the number of injections of each concentration that you may need for a given dose:
|
- Remove the box with the syringe from the refrigerator and let it sit unopened for approximately 30 minutes, until it reaches room temperature (leave the syringe in the box to protect it from light).
- When you are ready to use the syringe, wash your hands well with soap and water.
- Disinfect the injection site well with an alcohol swab.
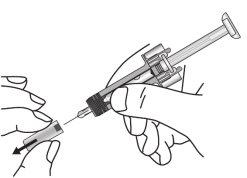
- Remove the plastic tray from the box and remove the paper covering it. Hold the syringe by the middle of its blue shield and pull the syringe out of the tray.
- Inspect the syringe. The liquid should be clear to slightly cloudy. Its color may vary from colorless to pale yellowish-brown. There may be a small air bubble, which is normal. DO NOT USE if the syringe is broken or if the liquid is clearly cloudy, has a clearly brown color, or contains particles. In all these cases, return the complete package to the pharmacy.
- Hold the syringe horizontally to check the expiry date printed on the label through the viewer. Note: You can rotate the inner part of the syringe so that the label can be read through the viewer window. DO NOT USE if the medicine has expired. If it has expired, return the complete package to the pharmacy.
Using the Xolair Pre-filled Syringe Injectable Solution
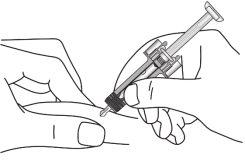
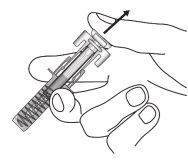
| Carefully remove the needle cap from the syringe. Discard the cap. You may see a drop at the tip of the needle. This is normal. |
| Gently pinch the skin at the injection site and insert the needle as shown. Insert the needle all the way to ensure that all the medicine is administered. |
| Hold the syringe as shown. Slowly press the plunger all the way until the plunger head is engaged in the activation clips of the shield. |
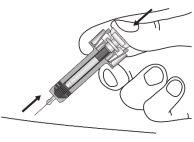
| Keep the plunger fully pressedwhile carefully removing the needle from the injection site. |
| Release the plunger slowly and let the syringe shield automatically cover the needle. You may see a little blood at the injection site. You can press the injection site with a cotton ball or gauze for 30 seconds. Do not rub the injection site. You can put a band-aid if you need to. |
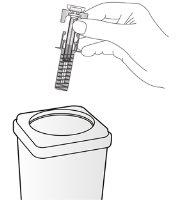
Disposal Instructions
| Discard the used syringe in a sharps disposal container (a closed and puncture-resistant container). For safety and health reasons (yours and others), used needles and syringes should never be reused. The disposal of unused medicine and all materials that have come into contact with it will be carried out in accordance with local regulations. Medicines should not be thrown down the drain or into the trash. Ask your pharmacist how to dispose of the packaging and medicines you no longer need. This will help protect the environment. |
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to XOLAIR 150 mg INJECTABLE SOLUTIONDosage form: INJECTABLE, 150 mgActive substance: omalizumabManufacturer: Celltrion Healthcare Hungary Kft.Prescription requiredDosage form: INJECTABLE, 75 mgActive substance: omalizumabManufacturer: Celltrion Healthcare Hungary Kft.Prescription requiredDosage form: INJECTABLE, 150 mgActive substance: omalizumabManufacturer: Novartis Europharm LimitedPrescription required
Online doctors for XOLAIR 150 mg INJECTABLE SOLUTION
Discuss questions about XOLAIR 150 mg INJECTABLE SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions