VIGZIP 100 mg/ml ORAL SOLUTION

How to use VIGZIP 100 mg/ml ORAL SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Patient Information Leaflet
Vigzip 100 mg/ml Oral Solution
vigabatrin
Read this leaflet carefully before you start taking this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack:
- What is Vigzip and what is it used for
- What you need to know before taking Vigzip
- How to take Vigzip
- Possible side effects
- How to store Vigzip
- Contents of the pack and further information
1. What is Vigzip and what is it used for
Vigzip contains the active substance vigabatrin.
This medicine is used to help control various forms of epilepsy.
It is used in combination with your current medication to treat "hard-to-control" epilepsy. Initially, it will be prescribed by a specialist. Your response to treatment will be monitored.
It is also used to control infant spasms (West syndrome).
2. What you need to know before taking Vigzip
Do not take Vigzip
If you are allergic to vigabatrin or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Talk to your doctor before starting Vigzip if:
- you are breast-feeding
- you are pregnant or planning to become pregnant
- you have had depression or any other psychiatric disorder in the past
- you have had kidney problems
- you have had vision problems.

Loss of vision (loss of vision from the edges of your field of vision) may occur during treatment with vigabatrin. You should discuss this possibility with your doctor before starting treatment with this medicine. This loss of vision can be severe, leading to tunnel vision or loss of vision, and irreversible, so it must be detected early. It cannot be ruled out that this loss of vision may worsen after treatment is stopped. It is essential that you inform your doctor promptly if you notice any change in your vision. Your doctor will perform a visual field and visual acuity test before you start taking Vigzip and at regular intervals during treatment. Vigabatrin may cause decreased vision due to eye problems such as retinal disorders, blurred vision, optic atrophy, or optic neuritis (see section 4). If your vision changes, consult an ophthalmologist.
If you develop symptoms such as drowsiness, decreased consciousness and movement (stupor) or confusion, consult your doctor, who will decide whether to reduce the dose or stop treatment.
A small number of people treated with antiepileptics like vigabatrin have had thoughts of self-harm or suicide. If you have these thoughts at any time, contact your doctor immediately.
Children
There have been reports of movement disorders and abnormalities in magnetic resonance imaging (MRI) of the brain in young children treated for infant spasms (West syndrome). If you notice unusual movement disorders in your child, consult your doctor, who will decide whether to consider changing treatment.
Other medicines and VIGZIP
Tell your doctor or pharmacist if you are taking, have recently taken, or might take any other medicines.
Tell your doctor if you are taking clonazepam, as concomitant use with Vigzip may increase the risk of sedation.
Vigzip should not be used in combination with other medicines that may have eye-related adverse effects.
Pregnancy, breast-feeding, and fertility
If you are pregnant or breast-feeding, think you may be pregnant, or plan to become pregnant, consult your doctor before using this medicine.
Do not take Vigzip during pregnancy unless your doctor tells you to. This medicine may cause problems to the fetus. However, do not stop treatment abruptly, as this may put the health of the mother and baby at risk.
Vigabatrin passes into breast milk. If you are breast-feeding, ask your doctor before using this medicine. You should not breast-feed during treatment.
Driving and using machines
Do not drive or operate machinery if your epilepsy is not controlled.
This medicine may cause symptoms such as drowsiness, dizziness, or changes in vision, and may reduce your reaction ability. These effects, as well as the disease itself, may make it difficult for you to drive vehicles or operate machinery. Therefore, do not drive, operate machinery, or engage in other activities that require special attention until your doctor assesses your response to this medicine.
Some patients taking this medicine have experienced visual disturbances that may affect their ability to drive or operate machinery. If you want to continue driving, you should undergo regular examinations (every six months) to rule out visual disturbances, even if you do not notice any changes in your vision.
Vigzip contains methyl parahydroxybenzoate (E 218) and propyl parahydroxybenzoate (E 216)
which may cause allergic reactions (possibly delayed).
3. How to take Vigzip
Follow the instructions for administration of this medicine exactly as indicated by your doctor. In case of doubt, consult your doctor or pharmacist.
It is essential to follow your doctor's instructions exactly. Never change the dose. The doctor prescribes the dose and adjusts it individually for each patient.
The recommended initial dose for adults is 1 g of vigabatrin (10 ml of solution) per day. However, your doctor may increase or decrease the dose depending on your response to treatment. The usual daily dose for adults is 2 to 3 g of vigabatrin (20 ml to 30 ml of solution). The maximum recommended dose is 3 g/day.
The daily dose can be taken as a single dose or divided into two doses.
If you are an elderly patient and/or have kidney problems, your doctor will prescribe a lower dose.
Pediatric use
Resistant partial epilepsy
In children, the dose is based on age and weight. The recommended initial dose for children is 40 milligrams per kilogram of body weight per day. The table below indicates the number of milliliters to be administered to a child depending on their body weight. Remember that this is only a guide. The child's doctor will decide if a slightly different dose is prescribed.
Body weight: | 10 to 15 kg: | 0.5-1 g/day | 5 to 10 ml solution/day |
15 to 30 kg: | 1-1.5 g/day | 10 to 15 ml solution/day | |
30 to 50 kg: | 1.5-3 g/day | 15 to 30 ml solution/day | |
over 50 kg: | 2 to 3 g/day (adult dose) | 20 to 30 ml solution/day (adult dose) |
Children with infant spasms (West syndrome)
The recommended initial dose for infants with West syndrome (infant spasms) is 50 milligrams of vigabatrin per kilogram of body weight per day, although sometimes higher doses may be used.
Method of administration
Vigzip is for oral use
This medicine can be taken before or after meals
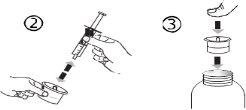
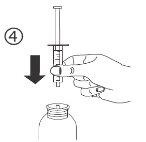
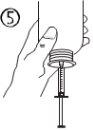
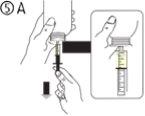
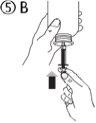
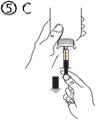
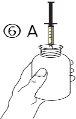
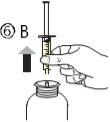
The pack contains an oral syringe (10 ml with graduations every 0.25 ml after the first 1.0 ml) and an adapter.
A full oral syringe (10 ml) is equivalent to 1,000 mg (1 g) of vigabatrin. The minimum volume that can be withdrawn is 1.0 ml (100 mg of vigabatrin). Volumes greater than 1.0 ml can be withdrawn in increments of 0.25 ml, which is equivalent to 25 mg of vigabatrin.
Instructions for use |
|
|
|
|
|
|
|
|
|
|
|
|
For volumes greater than 10 ml, repeat steps 4 to 6 until the complete dose has been administered. |
|
If you take moreVigzip than you should
If you or your child accidentally take too much Vigzip or too many doses, consult your doctor immediately or go to the hospital or nearest Poison Information Service.
Possible symptoms of overdose include drowsiness or loss/decreased level of consciousness.
In case of overdose or accidental ingestion, consult your doctor or call the Poison Information Service, phone 91 562 04 20, indicating the medicine and the amount ingested.
If you forget to take VIGZIP
If you forget to take a dose, take it as soon as you remember. If it is almost time for your next dose, take only one dose. Do not take a double dose to make up for the forgotten dose.
If you stop treatment with VIGZIP
Do not stop treatment prematurely without consulting your doctor first. If your doctor decides to stop your treatment, they will give you recommendations on how to gradually reduce the dose. Do not stop it abruptly, as this may cause your seizures to return.
If you have any doubts about the use of this medicine, consult your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Some patients may experience an increase in the number of seizures (convulsions) while taking this medicine. If this happens to you or your child, contact your doctor immediately.
Tell your doctor immediately if you experience:
- Visual field disturbances: About 1/3 or 33 out of 100 patients treated with vigabatrin may experience changes in their visual field (narrowing of the visual field). This "visual field defect" can be mild to severe. It is usually detected after months or years of treatment with vigabatrin. Visual field changes can be irreversible, so it is essential to detect them early. If you or your child experience any visual disturbances, contact your doctor or hospital immediately (very common: may affect more than 1 in 10 people).
Other side effects include:
Very common(may affect more than 1 in 10 people)
- Excessive fatigue and drowsiness
- Joint pain.
Common(may affect up to 1 in 10 people)
- Headache
- Weight gain
- Tremor
- Swelling (edema)
- Dizziness
- Feeling of numbness or tingling
- Concentration and memory disturbances
- Psychological disturbances, including agitation, aggression, nervousness, irritability, depression, thought disturbances, and unfounded suspicions (paranoia), insomnia. These side effects are usually reversible with dose reduction or gradual discontinuation of treatment. However, do not reduce your dose without consulting your doctor first. Contact your doctor if you experience these side effects.
- Nausea, vomiting, and abdominal pain
- Blurred vision, double vision, and rapid involuntary eye movements, which can cause dizziness
- Speech disorders
- Decreased red blood cell count (anemia)
- Unusual hair loss or weakened hair (alopecia).
Uncommon(may affect up to 1 in 100 people)
- Lack of coordination or clumsiness
- More severe psychological disorders, such as excitement or over-excitement, leading to unusual behaviors, disconnected from reality
- Skin rash.
Rare(may affect up to 1 in 1,000 people)
- Severe allergic reaction, which causes swelling of the face or throat. If you experience any of these symptoms, inform your doctor immediately.
- Hives
- Marked sedation, stupor, and confusion. These side effects are usually reversible with dose reduction or gradual discontinuation of treatment. However, do not reduce your dose without consulting your doctor first. Contact your doctor if you experience these side effects.
- Suicidal attempt
- Other eye disorders, such as retinal disorder, for example, poor night vision, and difficulty adjusting from bright to darker areas, sudden unexplained loss of vision, loss of vision in the edges of the visual field, sensitivity to light.
Very rare(may affect up to 1 in 10,000 people)
- Other eye disorders, such as eye pain (optic neuritis) and vision loss, including color vision loss (optic atrophy)
- Hallucinations (feeling, seeing, or hearing things that are not there)
- Liver disorders.
Frequency not known(frequency cannot be estimated from available data)
- Decreased visual acuity
- Abnormal changes in brain images taken by MRI
- Swelling of the protective layer of nerve cells in the brain observed in MRI images
Additional side effects in children
Very common(may affect more than 1 in 10 people)
- Excitement or restlessness.
Frequency not known(frequency cannot be estimated from available data)
- Movement disorders in young children treated for infant spasms
- Abnormal changes in brain images taken by MRI, especially in infants
- Swelling of the protective layer of nerve cells in the brain observed in MRI images, especially in infants.
Reporting of side effects
If you experience side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report them directly through the Spanish Pharmacovigilance System for Human Use Medicines: www.notificaRAM.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Vigzip
- Keep this medicine out of the sight and reach of children.
- Do not use this medicine after the expiry date stated on the packaging and label of the bottle after EXP. The expiry date is the last day of the month indicated.
- Do not store above 30°C.
- Discard 90 days after first opening the bottle. Do not store above 25°C after first opening the bottle.
- Do not use this medicine if you notice that the solution has changed color or shows signs of deterioration. Consult your pharmacist.
- Medicines should not be disposed of via wastewater or household waste. Place the packaging and any unused medicine in the SIGRE collection point at your usual pharmacy. Ask your pharmacist how to dispose of medicines no longer needed. This will help protect the environment.
6. Packaging Content and Additional Information
Composition ofVigzip
The active ingredient is vigabatrin.
Each ml contains 100 mg of vigabatrin.
The other ingredients are methyl parahydroxybenzoate (E 218), propyl parahydroxybenzoate (E 216), sucralose, and peppermint flavor (contains menthofuran, pulegone, estragole).
Product Appearance and Packaging Content
Oral solution
Vigzip is a clear, colorless to pale yellow solution.
HDPE bottle with child-resistant closure, containing 150 ml of oral solution.
The packaging contains a 10 ml oral syringe with printed marks at 1.0 ml, 2.0 ml, 3.0 ml, 4.0 ml, 5.0 ml, 6.0 ml, 7.0 ml, 8.0 ml, 9.0 ml, and 10.0 ml, and graduations at every 0.25 ml after the 1.0 ml mark; along with a bottle adapter.
Marketing Authorization Holder
RIA Generics Limited
The Black Church
St. Mary’s Place, Dublin 7
D07 P4AX, Ireland
Manufacturer
RIA Generics Limited
Cube Building, Monahan Road,
Cork, T12 H1XY, Ireland
Date of the Last Revision of this Leaflet: 02/2024
Detailed information on this medicinal product is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to VIGZIP 100 mg/ml ORAL SOLUTIONDosage form: TABLET, 100 mgActive substance: vigabatrinManufacturer: Orphelia PharmaPrescription requiredDosage form: TABLET, 500 mgActive substance: vigabatrinManufacturer: Orphelia PharmaPrescription requiredDosage form: TABLET, 500 mgActive substance: vigabatrinManufacturer: Sanofi Aventis S.A.Prescription required
Online doctors for VIGZIP 100 mg/ml ORAL SOLUTION
Discuss questions about VIGZIP 100 mg/ml ORAL SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions