How to use VIGAMOX 5 mg/ml EYE DROPS SOLUTION
Introduction
Package Leaflet: Information for the User
VIGAMOX 5mg/ml eye drops solution
Moxifloxacin (as hydrochloride)
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Vigamox and what is it used for
- What you need to know before you use Vigamox
- How to use Vigamox
- Possible side effects
- Storage of Vigamox
- Contents of the pack and other information
1. What is Vigamox and what is it used for
Vigamox contains the active substance moxifloxacin. Moxifloxacin belongs to a class of antibiotics called fluoroquinolones, which are used to treat bacterial infections of the eye.
Vigamox eye drops are used to treat eye infections (conjunctivitis) caused by bacteria.
Antibiotics are used to treat bacterial infections and do not work for viral infections.
It is important that you follow the instructions regarding dose, administration interval, and treatment duration as indicated by your doctor.
Do not store or reuse this medication. If you have any leftover antibiotic after completing treatment, return it to the pharmacy for proper disposal. Do not throw away medications down the drain or in the trash.
2. What you need to know before you use Vigamox
Do not useVigamox
- if you are allergic (hypersensitive) to moxifloxacin, to other quinolones, or to any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Consult your doctor or pharmacist:
- If you experience an allergic reaction to Vigamox. Allergic reactions occur rarely and serious reactions very rarely. If you experience any allergic reaction (hypersensitivity) or any side effect, see section 4.
- If you wear contact lenses, if you have signs or symptoms of an eye infection, stop wearing contact lenses and wear glasses. Do not wear contact lenses until the signs and symptoms of infection have resolved and you have finished using the medication.
- Tendon inflammation and rupture have been observed in people using oral or intravenous fluoroquinolones, especially in older patients and those treated concomitantly with corticosteroids. Discontinue Vigamox if you experience pain or inflammation of the tendons (tendinitis).
As with any antibiotic, long-term use of Vigamox may lead to other infections.
Other medicines and Vigamox
Tell your doctor or pharmacistif you are using, have recently used, or might use any other medicines, including those obtained without a prescription.
Pregnancy, breastfeeding, and fertility
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medicine.
Driving and using machines
You may experience blurred vision immediately after using Vigamox. Do not drive or use machines until these effects have resolved.
3. How to use Vigamox
Follow exactly the administration instructions of this medicine as indicated by your doctor. If in doubt, consult your doctor or pharmacist again.
The recommended dose is:
Adults, including elderly patients and children: 1dropin the affected eye(s), 3times a day(morning, afternoon, and evening).
Vigamox can be used in children, in patients over 65 years of age, and in patients with kidney or liver problems. The information on the use of this medicine in newborns is very limited, and therefore, its use is not recommended in these patients.
This medicine should only be applied to both eyes if your doctor has recommended it. Vigamox should onlybe used as eye drops.
The infection usually improves within 5 days. If you do not observe improvement, consult your doctor. You should continue using the drops for 2-3 days more or for the entire period of time indicated by your doctor.

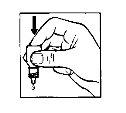

1 2 3
- Take the Vigamox bottle and stand in front of a mirror
- Wash your hands
- Remove the cap
- After opening the bottle for the first time, remove the plastic ring from the seal if it is loose
- Hold the bottle, upside down, between your thumb and the rest of your fingers
- Tilt your head back. Gently pull your lower eyelid down to form a pouch, where the drop should fall (figure1)
- Bring the tip of the bottle close to your eye. You may find it helpful to look in a mirror
- Do not touch the eye, eyelid, or surrounding areas with the dropperbecause the drops could become infected
- Gently squeeze the base of the bottle to release one drop of medicine at a time (figure2)
- After using Vigamox, press the edge of your eye, next to your nose, for 2-3 minutes(figure3). This helps prevent the medicine from entering the rest of your body and is especially important in young children
- If you are applying drops to both eyes, wash your hands before repeating the above steps for the other eye.This prevents the spread of infection from one eye to the other
- Close the bottle tightly immediately after use
If a drop falls outside the eye, try again.
If you have applied more medicinethan you should, you can remove it by rinsing your eyes with lukewarm water. Do not apply more drops until it is time for your next dose.
If you accidentally swallow Vigamox, contact your doctor or pharmacist.
If you forget to use the medicine, continue with the next scheduled dose. Do notapply a double dose to make up for the forgotten dose.
If you are using other eye drops, wait at least 5 minutes between applying Vigamox and the other drops.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, Vigamox can cause side effects, although not everybody gets them.
Unless the effects are severe or you experience a serious allergic reaction, continue with the treatment as usual.
If you experience a serious allergic reaction and any of the following symptoms appear:swelling of the hands, feet, ankles, face, lips, mouth, or throat that may cause difficulty swallowing or breathing, rash or hives, large blisters filled with fluid, sores, and ulcers, stop using Vigamox immediatelyand contact your doctor immediately.
Common side effects
(may affect up to 1 in 10 people)
Eye effects:eye pain, eye irritation
Uncommon side effects
(may affect up to 1 in 100 people)
Eye effects:dry eye, eye itching, eye redness, inflammation or scarring of the eye surface, rupture of a blood vessel in the eye, abnormal sensation in the eye, abnormality, itching, redness, or swelling of the eyelid
Other effects:headache, bad taste
Rare side effects
(may affect up to 1 in 1,000 people)
Eye effects:corneal disorder, blurred or reduced vision, conjunctival inflammation or infection, eye fatigue, eye swelling
Other effects:vomiting, nose discomfort, sensation of a lump in the throat, decrease in iron in the blood, abnormal liver function test values, abnormal sensation on the skin, pain, throat irritation
Frequency not known
(cannot be estimated from the available data)
Eye effects:eye infection, clouding of the eye surface, corneal swelling, deposits on the eye surface, increased pressure in the eye, scratch on the eye surface, eye allergy, eye discharge, increased tear production, sensitivity to light
Other effects:shortness of breath, irregular heartbeat, dizziness, increased allergic symptoms, itching, rash, skin redness, nausea, and hives
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly through the Spanish Medicines Agency's website: https://www.notificaRAM.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Vigamox
Keep out ofthe sight and reach of children.
Do not use this medicine after the expiry date which is stated on the bottle and carton after “EXP”. The expiry date refers to the last day of the month shown.
This medicine does not require any special storage conditions.
To avoid infections, discard the bottle 4weeks after first opening.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help protect the environment.
6. Contents of the pack and other information
Composition of Vigamox
- The active substance is moxifloxacin. One ml of eye drops contains 5 mg of moxifloxacin (as moxifloxacin hydrochloride, 5.45 mg). One drop of eye drops contains 190 micrograms of moxifloxacin.
- The other ingredients are: sodium chloride, boric acid, and purified water. Small amounts of sodium hydroxide and hydrochloric acid are added to maintain normal acidity levels (pH levels).
Appearance and pack contents
This medicine is a liquid (a clear, yellowish-green solution) that comes in a carton containing a 5 ml plastic bottle with a screw cap.
Marketing authorisation holder
Novartis Farmacéutica, S.A.
Gran Vía de les Corts Catalanes, 764
08013 – Barcelona, Spain
Manufacturer
Novartis Farmacéutica S.A.
Gran Via de les Corts Catalanes, 764
08013 Barcelona, Spain
Novartis Manufacturing NV
Rijksweg 14
2870 Puurs-Sint-Amands
Belgium
Novartis Pharma GmbH
Roonstrasse 25
90429 Nuremberg, Germany
Siegfried El Masnou, S.A.
C/ Camil Fabra, 58
08320 El Masnou
Barcelona, Spain
This medicine is authorised in the Member States of the European Economic Area and in the United Kingdom (Northern Ireland) under the following names:
MOXIFLOXACIN ALCON
Germany
KANAVIG
Belgium
Luxembourg
VIGAMOX
Bulgaria
Cyprus
Czech Republic
Denmark
Spain
Estonia
Finland
Greece
Hungary
Iceland
Italy
Latvia
Lithuania
Malta
Netherlands
Poland
Portugal
Romania
Slovakia
Slovenia
Sweden
MOXIVIG
United Kingdom (Northern Ireland)
Date of last revision of this leaflet:August 2023
Detailed information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es

How much does VIGAMOX 5 mg/ml EYE DROPS SOLUTION cost in Spain ( 2025)?
The average price of VIGAMOX 5 mg/ml EYE DROPS SOLUTION in December, 2025 is around 6.87 EUR. Prices may vary depending on the region, pharmacy, and whether a prescription is required. Always check with a local pharmacy or online source for the most accurate information.
- Country of registration
- Average pharmacy price6.87 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to VIGAMOX 5 mg/ml EYE DROPS SOLUTIONDosage form: EYEDROP, 5 mg/mlActive substance: moxifloxacinManufacturer: Tiedra Farmaceutica S.L.Prescription requiredDosage form: EYEDROP, 5 mg/mlActive substance: moxifloxacinManufacturer: Omnivision GmbhPrescription requiredDosage form: EYEDROP, 5 mg/mlActive substance: moxifloxacinManufacturer: Omnivision GmbhPrescription required
Alternatives to VIGAMOX 5 mg/ml EYE DROPS SOLUTION in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to VIGAMOX 5 mg/ml EYE DROPS SOLUTION in Польша
Alternative to VIGAMOX 5 mg/ml EYE DROPS SOLUTION in Украина
Online doctors for VIGAMOX 5 mg/ml EYE DROPS SOLUTION
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for VIGAMOX 5 mg/ml EYE DROPS SOLUTION – subject to medical assessment and local rules.