UPTRAVI 1000 MCG FILM-COATED TABLETS

Ask a doctor about a prescription for UPTRAVI 1000 MCG FILM-COATED TABLETS

How to use UPTRAVI 1000 MCG FILM-COATED TABLETS
Introduction
Leaflet: Patient Information
Uptravi 200micrograms film-coated tablets
Uptravi 400micrograms film-coated tablets
Uptravi 600micrograms film-coated tablets
Uptravi 800micrograms film-coated tablets
Uptravi 1000micrograms film-coated tablets
Uptravi 1200micrograms film-coated tablets
Uptravi 1400micrograms film-coated tablets
Uptravi 1600micrograms film-coated tablets
selexipag
This medicinal product is subject to additional monitoring, which will allow for quick identification of new safety information. You can help by reporting any side effects you may get. See the end of section 4 for how to report side effects.
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet (see section 4).
Contents of the pack and other information
- What is Uptravi and what is it used for
- What you need to know before you take Uptravi
- How to take Uptravi
- Possible side effects
- Storage of Uptravi
- Contents of the pack and other information
1. What is Uptravi and what is it used for
Uptravi is a medicine that contains the active substance selexipag. It works in the blood vessels in a similar way to the natural substance prostacyclin, making them relax and widen.
Uptravi is used for the long-term treatment of pulmonary arterial hypertension (PAH) in adult patients who are not adequately controlled with other PAH medicines known as endothelin receptor antagonists and phosphodiesterase type 5 inhibitors. Uptravi may be used on its own if the patient is not a candidate for these medicines.
PAH is a disease characterized by high blood pressure that affects the blood vessels that carry blood from the heart to the lungs (the pulmonary arteries). In people with PAH, these arteries are narrower, so the heart has to work harder to pump blood. This can make you feel tired, dizzy, short of breath, or experience other symptoms.
Like prostacyclin, Uptravi widens the pulmonary arteries and reduces their stiffness. This makes it easier for the heart to pump blood through the pulmonary arteries. It relieves the symptoms of PAH and improves the course of the disease.
2. What you need to know before you take Uptravi
Do not take Uptravi
- if you are allergic to selexipag or any of the other ingredients of this medicine (listed in section 6).
- if you have any heart disorder, such as:
- reduced blood flow to the heart muscle (severe ischemic cardiopathy or unstable angina); symptoms may include chest pain
- heart attack in the last 6 months
- heart failure (decompensated heart failure) without strict medical supervision
- severe irregular heart rhythm
- heart valve defect (congenital or acquired) that makes the heart work with difficulty (not related to pulmonary hypertension)
- if you have had a stroke in the last 3 months, or any other event that reduces blood flow to the brain (e.g. transient ischemic attack)
- if you are taking gemfibrozil (a medicine used to lower blood fat levels)
Warnings and precautions
Tell your doctor or pharmacist before you start taking Uptravi if:
- you are taking medicines for high blood pressure
- you have low blood pressure associated with symptoms such as dizziness
- you have recently had significant blood loss or fluid loss (e.g. severe diarrhea or vomiting)
- you have thyroid gland problems
- you have severe kidney problems or are on dialysis
- you have severe liver problems
If you experience any of the above signs or your disease changes, tell your doctor immediately.
Children and adolescents
Do not give this medicine to children under 18 years of age, as Uptravi has not been evaluated in children.
Elderly patients
There is limited experience with Uptravi in patients over 75 years of age. Uptravi should be used with caution in patients of this age group.
Other medicines and Uptravi
Tell your doctor if you are taking, have recently taken, or might take any other medicines.
Taking other medicines may affect the way Uptravi works.
Tell your doctor or PAH specialist if you are taking any of the following medicines:
- Gemfibrozil (a medicine used to lower blood fat levels)
- Clopidogrel (a medicine used to prevent blood clots in coronary artery disease)
- Deferasirox (a medicine used to remove excess iron from the body)
- Teriflunomide (a medicine used to treat multiple sclerosis)
- Carbamazepine (a medicine used to treat certain types of epilepsy, neuralgia, or to help control severe behavioral disorders when other medicines do not work)
- Phenytoin (a medicine used to treat epilepsy)
- Valproic acid (a medicine used to treat epilepsy)
- Probenecid (a medicine used to treat gout)
- Fluconazole, rifampicin, or rifapentine (antibiotics used to treat infections)
Pregnancy and breastfeeding
Uptravi is not recommended during pregnancy and breastfeeding. If you are a woman who can become pregnant, you must use a reliable method of contraception while taking Uptravi. If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before taking this medicine.
Driving and using machines
Uptravi may cause side effects such as headaches and low blood pressure (see section 4), which may affect your ability to drive; your disease symptoms may also reduce your ability to drive.
3. How to take Uptravi
Treatment with Uptravi should be started and controlled by a doctor who has experience in the treatment of pulmonary arterial hypertension (PAH). Follow your doctor's instructions for taking this medicine exactly. If you are unsure, consult your doctor again.
Tell your doctor if you experience side effects, as they may recommend that you change your Uptravi dose.
Tell your doctor if you are taking other medicines, as they may recommend that you take Uptravi only once a day.
If you have poor vision or experience any type of blindness, ask someone else to help you take Uptravi during the dose adjustment period.
Adjusting the right dose for you
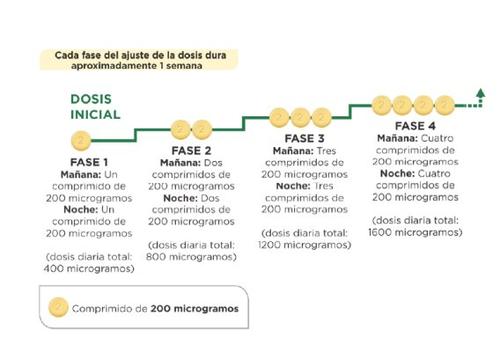
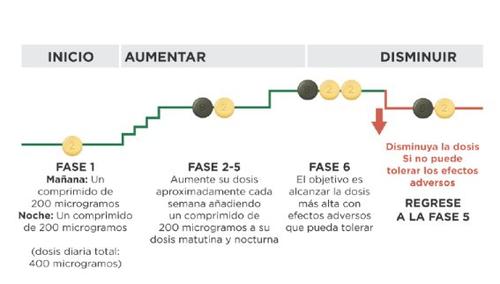
When you start treatment, you will take the lowest dose. This is one 200 microgram tablet in the morning and one 200 microgram tablet in the evening. You should start treatment in the evening. Your doctor will tell you to gradually increase the dose. This is called dose adjustment, and it allows your body to get used to the new medicine. The goal of dose adjustment is to reach the right dose for you. This will be the highest dose you can tolerate, which may be up to 1600 micrograms in the morning and 1600 micrograms in the evening.
The first pack of tablets you receive will contain light yellow 200 microgram tablets.
Your doctor will tell you to increase the dose in stages, usually every week, although the interval between increases may be longer.
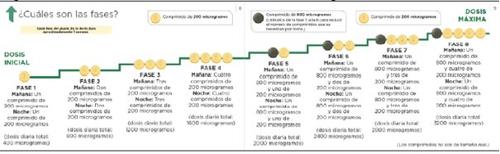
At each stage, you will add one 200 microgram tablet to your morning dose and one 200 microgram tablet to your evening dose. The first take of the increased dose should be done in the evening. The diagram below shows the number of tablets you should take each morning and each eveningin the first 4 stages.
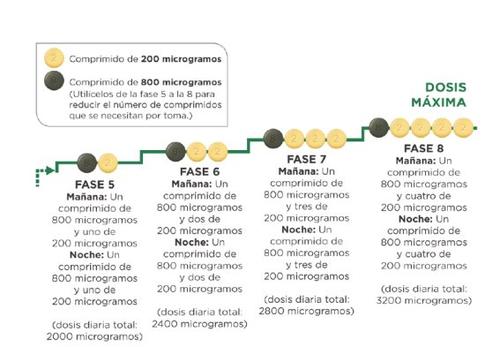
If your doctor tells you to continue increasing the dose and proceed to stage 5, you can do so by taking one green 800 microgram tablet and one light yellow 200 microgram tablet in the morning and one 800 microgram tablet and one 200 microgram tablet in the evening.
If your doctor tells you to continue increasing the dose, you will add one 200 microgram tablet to your morning dose and one 200 microgram tablet to your evening dose at each new stage. The first take of the increased dose should be done in the evening. The maximum dose of Uptravi is 1600 micrograms in the morning and 1600 micrograms in the evening. However, not all patients will reach this dose, as each patient requires a different dose.
The diagram below shows the number of tablets you should take each morning and each eveningat each stage, starting from stage 5.
The dose adjustment pack also contains a guide that provides information on the dose adjustment process and allows you to record the number of tablets you take daily.
Remember to record the number of tablets you take each day in your dose adjustment diary. The adjustment stages usually last about 1 week. If your doctor tells you to extend each adjustment stage beyond 1 week, you have additional pages in the diary to do so. Remember to communicate with your PAH specialist doctor or nurse periodically during the dose adjustment phase.
Dose reduction due to side effects
During dose adjustment, you may experience side effects such as headache, diarrhea, feeling unwell (nausea), being sick (vomiting), jaw pain, muscle pain, pain in the lower limbs, joint pain, or facial redness (see section 4). If these side effects are difficult to tolerate, consult your doctor about how to control or treat them. There are treatments available to help you alleviate these side effects. For example, painkillers like paracetamol can help you treat pain and headache.
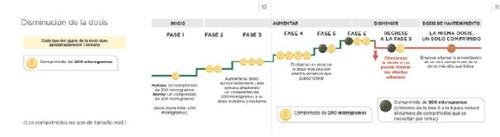
If the side effects cannot be treated, or they do not improve gradually with the dose you are taking, your doctor may adjust the dose by reducing the number of light yellow 200 microgram tablets you take, removing one tablet in the morning and one in the evening. The scheme below shows how to reduce the dose. This should only be done if your doctor tells you to.
If the side effects you experience can be controlled after dose reduction, your doctor may decide that you should maintain that dose. For more information, see the Maintenance dose section below.
Maintenance dose
The highest dose you can tolerate during the dose adjustment phase will become your maintenance dose. Your maintenance dose is the dose you should continue to take regularly.
Your doctor will prescribe a single tablet with the right strength for your maintenance dose. This allows you to take one tablet in the morning and one in the evening, instead of several tablets each time.
To see the full description of Uptravi tablets, including colors and engraving, see section 6 in this leaflet.
Over time, your doctor may adjust your maintenance dose if necessary.
If at any time, after taking the same dose for a long period, you experience side effects that you cannot tolerate or side effects that affect your daily activities, contact your doctor, as you may need a dose reduction. Your doctor may then prescribe a single tablet with a lower concentration. Remember to dispose of unused tablets (see section 5).
Take Uptravi once in the morning and once in the evening, with an interval of approximately 12 hours.
Take the tablets with food, as this can help you tolerate the medicine better. Swallow the tablets whole with a glass of water.
If you take more Uptravi than you should
If you take more tablets than you should, consult your doctor immediately.
If you forget to take Uptravi
If you forget to take Uptravi, take a dose as soon as you remember, and then continue taking the tablets at the usual time. If it is almost time for your next dose (within 6 hours of the time you usually take it), you should skip the missed dose and continue taking the medicine at the usual time. Do not take a double dose to make up for the missed dose.
If you stop taking Uptravi
Suddenly stopping treatment with Uptravi may make your symptoms worse. Do not stop taking Uptravi unless your doctor tells you to. Your doctor may tell you to gradually reduce the dose before stopping treatment completely.
If, for any reason, you stop taking Uptravi for more than 3 consecutive days (if you miss 3 morning doses and 3 evening doses, or 6 consecutive doses or more), contact your doctor immediately, as you may need to adjust the dose to avoid side effects. Your doctor may decide to restart treatment at a lower dose, to gradually increase it until you reach your maintenance dose.
If you have any other questions about the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, Uptravi can cause side effects. You may experience side effects not only during the dose adjustment phase, during which your dose is being increased, but also later, after you have been taking the same dose for a long time.
If you experience any of the following side effects: headache, diarrhea, feeling unwell (nausea), being sick (vomiting), jaw pain, muscle pain, pain in the lower limbs, joint pain, or facial redness that you cannot tolerate or are not treatable, you should contact your doctor, as the dose you are taking may be too high for you and you may need a dose reduction.
Very common side effects(may affect more than 1 in 10 people)
- Headache
- Flush (facial redness)
- Nausea and vomiting
- Diarrhea
- Jaw pain, muscle pain, joint pain, pain in the lower limbs
- Nasopharyngitis (nasal congestion)
Common side effects(may affect up to 1 in 10 people)
- Anemia (low red blood cell count)
- Hyperthyroidism (overactive thyroid gland)
- Decreased appetite
- Weight loss
- Hypotension (low blood pressure)
- Stomach pain
- Pain
- Changes in some laboratory test results, including those that measure blood cell counts and thyroid function
- Rashes, including urticaria, which can cause a burning or itching sensation and redness of the skin
Uncommon side effects(may affect up to 1 in 100 people)
Increased heart rate
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist, even if they are not listed in this leaflet. You can also report side effects directly through the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Uptravi
Keep this medicine out of the sight and reach of children.
Do not use Uptravi after the expiry date which is stated on the carton and on the blister after "EXP". The expiry date is the last day of the month shown.
This medicine does not require any special storage conditions.
No special precautions for disposal are necessary.
6. Package Contents and Additional Information
Composition of Uptravi
- The active substance is selexipag.
Uptravi 200 micrograms film-coated tablets contain 200 micrograms of selexipag
Uptravi 400 micrograms film-coated tablets contain 400 micrograms of selexipag
Uptravi 600 micrograms film-coated tablets contain 600 micrograms of selexipag
Uptravi 800 micrograms film-coated tablets contain 800 micrograms of selexipag
Uptravi 1,000 micrograms film-coated tablets contain 1,000 micrograms of selexipag
Uptravi 1,200 micrograms film-coated tablets contain 1,200 micrograms of selexipag
Uptravi 1,400 micrograms film-coated tablets contain 1,400 micrograms of selexipag
Uptravi 1,600 micrograms film-coated tablets contain 1,600 micrograms of selexipag
- The other ingredients are:
In the core of the tablets:
Mannitol (E421), corn starch, low-substituted hydroxypropylcellulose, hydroxypropylcellulose, and magnesium stearate.
In the film coating:
Hypromellose, propylene glycol, titanium dioxide (E171), carnauba wax, and iron oxides (see below).
Uptravi 200 micrograms film-coated tablets contain yellow iron oxide (E172).
Uptravi 400 micrograms film-coated tablets contain red iron oxide (E172).
Uptravi 600 micrograms film-coated tablets contain red and black iron oxide (E172).
Uptravi 800 micrograms film-coated tablets contain yellow and black iron oxide (E172).
Uptravi 1,000 micrograms film-coated tablets contain red and yellow iron oxide (E172).
Uptravi 1,200 micrograms film-coated tablets contain black and red iron oxide (E172).
Uptravi 1,400 micrograms film-coated tablets contain yellow iron oxide (E172).
Uptravi 1,600 micrograms film-coated tablets contain black, red, and yellow iron oxide (E172).
Appearance of Uptravi and Package Contents
Uptravi 200 micrograms film-coated tablets: Light yellow, round, film-coated tablets marked with a "2" on one side.
Uptravi 400 micrograms film-coated tablets: Red, round, film-coated tablets marked with a "4" on one side.
Uptravi 600 micrograms film-coated tablets: Light violet, round, film-coated tablets marked with a "6" on one side.
Uptravi 800 micrograms film-coated tablets: Green, round, film-coated tablets marked with an "8" on one side.
Uptravi 1,000 micrograms film-coated tablets: Orange, round, film-coated tablets marked with a "10" on one side.
Uptravi 1,200 micrograms film-coated tablets: Dark violet, round, film-coated tablets marked with a "12" on one side.
Uptravi 1,400 micrograms film-coated tablets: Dark yellow, round, film-coated tablets marked with a "14" on one side.
Uptravi 1,600 micrograms film-coated tablets: Brown, round, film-coated tablets marked with a "16" on one side.
Uptravi 200 micrograms film-coated tablets are supplied in blister packs containing 10 or 60 tablets and 60 or 140 tablets (dose adjustment packs).
Uptravi 400 micrograms, 600 micrograms, 800 micrograms, 1,000 micrograms, 1,200 micrograms, 1,400 micrograms, and 1,600 micrograms film-coated tablets are supplied in blister packs containing 60 tablets.
Not all pack sizes may be marketed.
Marketing Authorisation Holder
Janssen-Cilag International NV
Turnhoutseweg 30
B-2340 Beerse
Belgium
Manufacturer
Actelion Manufacturing GmbH
Emil-Barell-Strasse 7
79639 Grenzach-Wyhlen
Germany
Actelion Pharmaceuticals Belgium NV
Bedrijvenlaan 1
2800 Mechelen
Belgium
You can request more information about this medicinal product from the local representative of the marketing authorisation holder:
België/Belgique/Belgien Actelion, a division of Janssen-Cilag International NV Tel: +32-(0)15 284 777 | Lietuva Actelion, a division of Janssen-Cilag International NV Tel: +370 5 278 68 88 |
България Actelion, a division of Janssen-Cilag International NV Tel: +359 2 489 94 00 | Luxembourg/Luxemburg Actelion, a division of Janssen-Cilag International NV Tel: +32-(0)15 284 777 |
Ceská republika Actelion, a division of Janssen-Cilag International NV Tel: +420 221 968 006 | Magyarország Actelion, a division of Janssen-Cilag International NV Tel: +36-1-413-3270 |
Danmark Actelion, a division of Janssen-Cilag International NV Tel: +45 3694 45 95 | Malta Actelion, a division of Janssen-Cilag International NV Tel: +356 2397 6000 |
Deutschland Actelion, a division of Janssen-Cilag International NV Tel: +49 761 45 64 0 | Nederland Actelion, a division of Janssen-Cilag International NV Tel: +31 (0)348 435950 |
Eesti Actelion, a division of Janssen-Cilag International NV Tel: +372 617 7410 | Norge Actelion, a division of Janssen-Cilag International NV Tel: +47 22480370 |
Ελλάδα Actelion, a division of Janssen-Cilag International NV Tel: +30 210 675 25 00 | Österreich Actelion, a division of Janssen-Cilag International NV Tel: +43 1 505 4527 |
España Actelion, a division of Janssen-Cilag International NV Tel: +34 93 366 43 99 | Polska Actelion, a division of Janssen-Cilag International NV Tel: +48 (22) 262 31 00 |
France Actelion, a division of Janssen-Cilag International NV Tel: +33 (0)1 55 00 26 66 | Portugal Actelion, a division of Janssen-Cilag International NV Tel: +351 214 368 600 |
Hrvatska Actelion, a division of Janssen-Cilag International NV Tel: + 385 1 6610 700 | România Actelion, a division of Janssen-Cilag International NV Tel: + 40 21 207 1800 |
Ireland Actelion, a division of Janssen-Cilag International NV Tel: +353 1 800 709 122 | Slovenija Actelion, a division of Janssen-Cilag International NV Tel: +386 1 401 18 00 |
Ísland Actelion, a division of Janssen-Cilag International NV Tel: +46 8 544 982 50 | Slovenská republika Actelion, a division of Janssen-Cilag International NV Tel: +420 221 968 006 |
Italia Actelion, a division of Janssen-Cilag International NV Tel: +39 0542 64 87 40 | Suomi/Finland Actelion, a division of Janssen-Cilag International NV Tel: +358 9 2510 7720 |
Κύπρος Actelion, a division of Janssen-Cilag International NV Tel: +30 210 675 25 00 | Sverige Actelion, a division of Janssen-Cilag International NV Tel: +46 8 544 982 50 |
Latvija Actelion, a division of Janssen-Cilag International NV Tel: +371 678 93561 | United Kingdom Janssen-Cilag Ltd. Tel: +44 1 494 567 444 |
Date of Last Revision of this Leaflet: November 2018
Detailed information on this medicinal product is available on the European Medicines Agency website: http://www.ema.europa.eu.
DOSAGE ADJUSTMENT GUIDE: DOSAGE ADJUSTMENT PACK
Page 1
Uptravi film-coated tablets selexipag Dosage adjustment guide Starting treatment with Uptravi Please read the accompanying leaflet before starting treatment. Tell your doctor if you experience side effects, as they may recommend that you change your Uptravi dose. Tell your doctor if you are taking other medications, as they may recommend that you take Uptravi only once a day. |
Page 2
Page 3
Index How to take Uptravi?..................................................4 How to increase the dose?............................................6 What are the phases?...................................................8 When should the dose be decreased?..............................10 Dose decrease..............................................12 | Switching to the maintenance dose.........................14 If you forget to take Uptravi..............................................16 If you stop treatment with Uptravi...................17 Dose adjustment diary ................................18 |
Page 4
Page 5
How to take Uptravi? Uptravi is a medication that should be taken in the morningand in the eveningfor the treatment of pulmonary arterial hypertension, also known as PAH. The initial dose of Uptravi is 200 micrograms in the morning and in the evening. The first intake of Uptravi should be done in the evening. You should take each dose with a glass of water, preferably during meals. | There are 2 phases of treatment with Uptravi: Dose adjustment During the first few weeks, your doctor will need your cooperation to find the most suitable dose of Uptravi for you. Your doctor may increase your dose from the initial dose. Your doctor may decrease your dose. This process is known as dose adjustment, and it allows your body to gradually adapt to the medication. Maintenance Once your doctor has found the right dose for you, this will be the dose you will take regularly. This is called the maintenance dose. |
Page 6
Page 7
How to increase the dose? Treatment will start at a dose of 200 micrograms in the morning and in the evening, and after discussing it with your doctor or nurse, the dose will be increased to the next phase. The first intake of the increased dose should be done in the evening.Each adjustment phase usually lasts approximately 1 week. It may take several weeks to find the right dose for you. The goal is to reach the most suitable dose for your treatment. This dose will be your maintenance dose. | Each patient with PAH is different. Not all patients will end up with the same maintenance dose. Some patients may take 200 micrograms in the morning and in the evening as a maintenance dose, while others may reach the maximum dose of 1,600 micrograms in the morning and in the evening. Others may reach a maintenance dose at some point between the two. The important thing is to reach the most suitable dose for your own treatment. |
Page 8
Page 9
|
Page 10
Page 11
When should the dose be decreased? As with all medications, you may experience side effects as you increase the dose of Uptravi. Consult your doctor or nurse if you experience side effects.There are treatments available to help you alleviate them. The most common side effects (may affect more than 1 in 10 people) that you may experience while taking Uptravi are:
See the leaflet for the full list of side effects and additional information. | If you cannot tolerate the side effects even after your doctor or nurse has tried to treat them, they may recommend that you decrease the dose. If your doctor or nurse tells you to decrease the dose, take one 200 microgram tablet less in the morning and one less in the evening. You should only decrease the dose after consulting with your doctor or nurse. This dose decrease process will help you find the right dose for you, also known as the maintenance dose. |
Page 12
Page 13
|
Page 14
Page 15
Switching to the maintenance dose The highest dose you can tolerate during the dose adjustment phase will become your maintenance dose.Your maintenance dose is the dose you should continue to take regularly. Your doctor or nurse will prescribe a single tablet with the equivalent strengthfor your maintenance dose. This allows you to take a single tablet in the morning and one in the evening, instead of several tablets for each dose. | For example, if your highest tolerated dose during the dose adjustment phase was 1,200 micrograms once in the morning and once in the evening:
Over time, your doctor or nurse may adjust your maintenance dose if necessary. |
Page 16
Page 17
If you forget to take Uptravi If you forget to take a dose, take it as soon as you remember, and then continue taking the tablets at the usual time. If you remember within 6 hours before the time you should take the next dose, you should not take the missed dose and continue taking the medication at the usual time. Do not take a double dose to make up for the missed dose. | If you stop treatment with Uptravi Do not stop taking Uptravi unless your doctor or nurse tells you to. If, for any reason, you stop taking Uptravi for more than 3 consecutive days (if you have missed 6 consecutive doses or more), contact your doctor or nurse immediately, as you may need to adjust the dose to avoid side effects. Your doctor or nurse may decide to restart treatment at a lower dose, to gradually increase it until you reach your previous maintenance dose. |
Page 18
Page 19
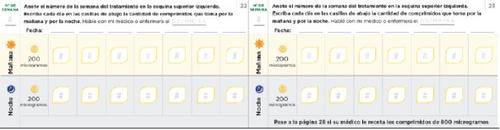
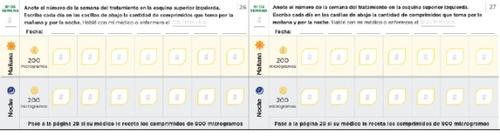
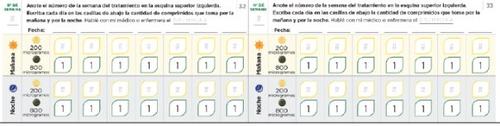
Dose adjustment diary Read the instructions in the leaflet carefully. The following pages of the diary will help you keep a record of the number of tablets you should take in the morning and in the evening during the dose adjustment phase. Use them to record the number of tablets you take in the morning and in the evening. Each phase usually lasts approximately 1 week, unless your doctor or nurse tells you otherwise. If the dose adjustment phases last more than one week, you have additional pages in your diary to record it. Use pages 20 to 27 to record the first weeks of treatment, when you receive only 200 microgram tablets (phases 1-4). If you have been prescribed both 200 and 800 microgram tablets, use pages 30 to 37 (phases 5-8). | Remember to communicate with your PAH specialist doctor or nurse regularly. Write down your doctor's or nurse's instructions: Doctor's phone number and email: Pharmacist's phone number: Notes: |
Page 20
Page 21
|
Page 23
|
Page 24Page 25
|
Page 26Page 27
|
Page 28Page 29
Use the following diary pages if your doctor or nurse prescribes 800 microgram tablets in addition to the 200 microgram tablets. In the diary pages, check that you have taken one800 microgram tablet every day in the morning and at night along with the number of 200 microgram tablets prescribed for you.
| Remember to communicate with your doctor or nurse specialist in HAP on a regular basis. Note your doctor's or nurse's instructions: Doctor's phone and email: Pharmacist's phone: Notes: |
Page 30Page 31
|
Page 32Page 33
|
Page 34Page 35
|
Page 36Page 37
|
Page 38Page 39
Notes |
Page 40
Actelion Pharmaceuticals Ltd. |
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to UPTRAVI 1000 MCG FILM-COATED TABLETSDosage form: TABLET, 1,200 microgramsActive substance: selexipagManufacturer: Janssen-Cilag International N.VPrescription requiredDosage form: TABLET, 1,400 microgramsActive substance: selexipagManufacturer: Janssen-Cilag International N.VPrescription requiredDosage form: TABLET, 1,600 microgramsActive substance: selexipagManufacturer: Janssen-Cilag International N.VPrescription required
Online doctors for UPTRAVI 1000 MCG FILM-COATED TABLETS
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for UPTRAVI 1000 MCG FILM-COATED TABLETS – subject to medical assessment and local rules.