
UPSTAZA 2.8 × 10^11 GENE THERAPY VECTORS (VG)/0.5 ML SOLUTION FOR INFUSION

How to use UPSTAZA 2.8 × 10^11 GENE THERAPY VECTORS (VG)/0.5 ML SOLUTION FOR INFUSION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Upstaza 2.8 × 1011vector genomes/0.5 ml solution for infusion
eladocagene exuparvovec
This medicinal product is subject to additional monitoring, which will allow for the quick identification of new safety information. You can help by reporting any side effects you or your child may experience. The last section of section 4 will provide information on how to report side effects.
Read all of this leaflet carefully before you or your child are given this medicine, because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or nurse.
- If you or your child experience any side effects, talk to your doctor or nurse, even if they are not listed in this leaflet. See section 4.
Contents of the pack
- What is Upstaza and what is it used for
- What you need to know before you or your child are given Upstaza
- How Upstaza is given to you or your child
- Possible side effects
- Storage of Upstaza
- Contents of the pack and other information
1. What is Upstaza and what is it used for
What is Upstaza
Upstaza is a gene therapy medicine that contains the active substance eladocagene exuparvovec.
What Upstaza is used for
Upstaza is used to treat patients aged 18 months and older with a deficiency of a protein called L-amino acid decarboxylase (AADC). This protein is essential for making certain substances that the body's nervous system needs to function properly.
AADC deficiency is a hereditary condition caused by a mutation (change) in the gene that controls the production of AADC (also called the dopa decarboxylase or DDC gene). This condition prevents the development of the child's nervous system, which means that many of the body's functions do not develop properly during childhood, such as movement, feeding, breathing, speech, and mental ability.
How Upstaza works
The active substance in Upstaza, eladocagene exuparvovec, is a type of virus called an adeno-associated virus that has been modified to include a copy of the working DDC gene. Upstaza is given by infusion (drip) into a part of the brain called the putamen, where AADC is made. The adeno-associated virus allows the DDC gene to enter the brain cells. This allows the cells to produce AADC so that the body can make the substances that the nervous system needs.
The adeno-associated virus used to deliver the gene does not cause disease in humans.
2. What you need to know before you or your child are given Upstaza
You or your child will not be given Upstaza:
- if you or your child are allergic to eladocagene exuparvovec or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
- Mild or moderate uncontrollable spasmodic movements (also called dyskinesia) or sleep disorders (insomnia) may appear or worsen 1 month after treatment with Upstaza and may last for several months. Your doctor will decide if you or your child need treatment for these effects.
- Your doctor will monitor you or your child to detect complications of treatment with Upstaza, such as leakage of the fluid that surrounds the brain, meningitis, or encephalitis.
- During the days following the procedure, your doctor will monitor your child for possible complications such as a result of the procedure and general anesthesia. Some symptoms of the disease may worsen during this period.
- Some specific symptoms of AADC deficiency may persist after treatment, such as effects on mood, sweating, and body temperature.
- After treatment, some of the medicine may pass into your or your child's body fluids (e.g., tears, blood, nasal secretions, and cerebrospinal fluid); this is known as "shedding". You or your child and the person caring for them (especially if they are pregnant, breastfeeding, or immunocompromised) should wear gloves and place used dressings and other waste materials with tears and nasal secretions in sealed bags before disposing of them. You should follow these precautions for 14 days.
- You or your child should not donate blood, organs, tissues, or cells for transplants after treatment with Upstaza, as Upstaza is a gene therapy medicine.
Children and adolescents
Upstaza has not been studied in children under 18 months of age. Experience is limited in children over 12 years of age.
Other medicines and Upstaza
Tell your doctor if you or your child are taking, have recently taken, or might take any other medicines.
You or your child can receive routine childhood vaccinations as usual.
Pregnancy, breastfeeding, and fertility
The effects of this medicine on pregnancy and the fetus are unknown.
Upstaza has not been studied in breastfeeding women.
There is no information on the effect of Upstaza on male or female fertility.
Upstaza contains sodium and potassium
This medicine contains less than 1 mmol of sodium (23 mg) per dose; this is essentially "sodium-free".
This medicine contains less than 1 mmol of potassium (39 mg) per dose; this is essentially "potassium-free".
3. How Upstaza is given to you or your child
- You or your child will receive Upstaza in the operating room by neurosurgeons with experience in brain surgery.
- Upstaza will be given under anesthesia. The neurosurgeon will talk to you about the anesthesia and how it is given.
- Before giving Upstaza, the neurosurgeon will make two small holes in your or your child's skull, one on each side.
- Then, Upstaza will be injected through these holes into four points in your or your child's brain, in an area called the putamen.
- After the infusion, the two holes will be closed and you or your child will undergo a brain scan.
- You or your child will need to stay in the hospital or near the hospital for a few days to monitor recovery and check for any side effects of the surgical procedure or anesthesia.
- Your doctor will see you or your child in the hospital twice, once about a week after the surgical procedure, and again three weeks after, to monitor recovery and check for any side effects of the surgical procedure and treatment.
If you or your child are given too much Upstaza
Since this medicine is given to you or your child by a doctor, it is unlikely that you or your child will receive too much. If this happens, your doctor will treat the symptoms as needed.
If you have any further questions on the use of this medicine, ask your doctor.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
The following side effects may occur with Upstaza:
Very common (may affect more than 1 in 10 people)
- Dyskinesia (uncontrollable spasmodic movements)
- Insomnia (difficulty sleeping), irritability
Common (may affect up to 1 in 10 people):
- Increased saliva production
The following side effects may occur with the surgical procedure to administer Upstaza:
Very common (may affect more than 1 in 10 people)
- Low red blood cell count (anemia)
- Leakage of the fluid that surrounds the brain (called cerebrospinal fluid) (possible symptoms are headache, nausea and vomiting, neck pain or stiffness, changes in hearing, feeling of imbalance, dizziness or vertigo)
The following side effects may occur in the two weeks following the surgical procedure to administer Upstaza, due to anesthesia or postoperative effects:
Very common (may affect more than 1 in 10 people)
- Gastrointestinal bleeding, diarrhea
- Fever, abnormal respiratory sounds
- Pneumonia
- Low potassium levels in the blood
- Irritability
- Low blood pressure (hypotension)
Common (may affect up to 1 in 10 people):
- Cyanosis (bluish discoloration of the skin due to lack of oxygen in the blood)
- Mouth ulcers
- Low body temperature (hypothermia)
- Gastroenteritis
- Dyskinesia (uncontrollable spasmodic movements)
- Respiratory failure
- Pressure ulcers, diaper dermatitis, skin rash
- Tooth extraction
- Hypovolemic shock (severe loss of blood or body fluids)
Reporting of side effects
If you or your child experience any side effects, talk to your doctor or nurse, even if they are not listed in this leaflet. You can also report side effects directly through the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Upstaza
The following information is intended only for healthcare professionals.
Upstaza will be stored in the hospital. It should be stored and transported frozen at a temperature of ≤ -65 °C. It is thawed before use and, once thawed, must be used within 6 hours. It must not be re-frozen.
Do not use this medicine after the expiry date which is stated on the carton after EXP.
6. Package Contents and Additional Information
Upstaza Composition
- The active substance is eladocagene exuparvovec. Each 0.5 ml solution contains 2.8 × 10^11 vector genomes of eladocagene exuparvovec.
The other components are potassium chloride, sodium chloride, potassium dihydrogen phosphate, disodium hydrogen phosphate, poloxamer 188, and water for injectable preparations (see section 2 "Upstaza contains sodium and potassium").
Product Appearance and Package Contents
Upstaza is a solution for infusion, clear or slightly opalescent, colorless or pale white, presented in a transparent glass vial.
Each package contains 1 vial.
Marketing Authorization Holder
PTC Therapeutics International Limited
70 Sir John Rogerson's Quay
Dublin 2
Ireland
Manufacturer
Almac Pharma Services (Ireland) Limited
Finnabair Industrial Estate
Dundalk, Co. Louth, A91 P9KD
Ireland
For further information about this medicinal product, please contact the local representative of the marketing authorization holder:
AT, BE, BG, CY, CZ, DK, DE, EE, EL, ES, HR, HU, IE, IS, IT, LT, LU, LV, MT, NL, NO, PL, PT, RO, SI, SK, FI, SE, UK (NI) PTC Therapeutics International Ltd. (Ireland) +353 (0)1 447 5165 | FR PTC Therapeutics France Tel: +33(0)1 76 70 10 01 |
Date of Last Revision of this Leaflet
This medicinal product has been authorized under "exceptional circumstances". This means that due to the rarity of this disease, it has not been possible to obtain complete information on this medicinal product.
The European Medicines Agency will review any new information that may become available every year and this leaflet will be updated as necessary.
Other Sources of Information
Detailed information on this medicinal product is available on the European Medicines Agency website: http://www.ema.europa.eu.
This information is intended for healthcare professionals only:
Instructions on preparation, administration, measures taken in case of accidental exposure, and disposal of Upstaza
Each vial is for single use. This medicinal product should only be injected with the SmartFlow ventricular cannula.
Precautions to be taken before handling or administering the medicinal product
This medicinal product contains genetically modified viruses. During preparation, administration, and disposal, personal protective equipment (including gown, safety glasses, mask, and gloves) should be used when handling eladocagene exuparvovec and materials that have come into contact with the solution (solid and liquid residues).
Thawing in the hospital pharmacy
- Upstaza is delivered to the pharmacy frozen and should be stored in the outer packaging at a temperature ≤ -65 °C until it is prepared for use.
- Upstaza should be handled aseptically and under sterile conditions.
- Allow the frozen vial of Upstaza to thaw at room temperature in an upright position until the contents are completely thawed. Gently invert the vial 3 times, DO NOT shake.
- Inspect Upstaza after mixing. If particles, turbidity, or color change are observed, do not use the product.
Preparation before administration
- Transfer the vial, syringe, needle, syringe cap, sterile bags, or sterile wrappers following the hospital procedure for transfer and use of the filled syringe in the planned operating room, and label it in the biological safety cabinet (BSC). Use sterile gloves and other personal protective equipment (including gown, safety glasses, and mask) according to the standard operating procedure for BSC.
- Open the 5 ml syringe (5 ml polypropylene syringe with latex-free elastomer plunger, lubricated with medical-grade silicone oil) and label it properly as a filled product syringe according to the pharmacy procedure and local regulations.
- Attach the 18 or 19 gauge needle with filter (5 µm stainless steel filter, 1.5 inches, 18 or 19 gauge) to the syringe.
- Insert the entire volume of the Upstaza vial into the syringe. Invert the vial and syringe and partially withdraw or tilt the needle as necessary to maximize product recovery.
- Aspirate air into the syringe so that the needle is empty of product. Carefully remove the needle from the 5 ml syringe containing Upstaza. Purge the air from the syringe until no air bubbles are present, then cap with a syringe cap.
- Wrap the syringe in a sterile plastic bag (or multiple bags according to the hospital's standard procedure) and place it in a suitable secondary container (e.g., a hard plastic cooler) to transport to the operating room at room temperature. The use of the syringe (i.e., connection of the syringe to the syringe dispenser and start of cannula priming) should begin within 6 hours of starting product thawing.
Administration in the operating room
- Firmly attach the syringe containing Upstaza to the SmartFlow ventricular cannula.
- Insert the Upstaza syringe into a compatible infusion pump. Infuse Upstaza at 0.003 ml/min until the first drop of Upstaza can be seen at the tip of the needle. Stop and wait until ready for infusion.
Precautions to be taken for disposal of the medicinal product and accidental exposure
- Accidental exposure to eladocagene exuparvovec should be avoided, including contact with skin, eyes, and mucous membranes.
- In case of skin exposure, the affected area should be thoroughly cleaned with soap and water for at least 5 minutes. In case of eye contact, the affected area should be thoroughly rinsed with water for at least 5 minutes.
- In case of needlestick injury, the affected area should be thoroughly cleaned with soap and water or a disinfectant.
- Any unused eladocagene exuparvovec or residual materials should be disposed of according to local regulations on pharmaceutical waste.
- Any spills should be cleaned up with absorbent gauze and disinfected with a bleach solution, followed by the use of alcohol wipes.
- After administration, the risk of dissemination is considered low. It is recommended to advise caregivers and patient family members to follow proper precautions for handling patient bodily fluids and waste for 14 days after administration of eladocagene exuparvovec (see section 4.4 of the summary of product characteristics).
Dosage
Treatment should be administered in a specialized neurosurgery center by a qualified neurosurgeon under controlled aseptic conditions.
Patients will receive a total dose of 1.8 × 10^11 vg administered in four infusions of 0.08 ml (0.45 × 10^11 vg) (two per putamen).
The dosage is the same for all populations included in the indication.
Method of administration
Intraputaminal route.
Administration of Upstaza may cause cerebrospinal fluid leakage after surgical intervention. Patients receiving treatment with Upstaza should be closely monitored after administration.
Neurosurgical administration
Upstaza is a single-use vial administered by bilateral intraputaminal injection in one surgical session at two points in the putamen. Four separate injections of equal volumes are applied to the right anterior putamen, right posterior putamen, left anterior putamen, and left posterior putamen.
Follow these steps to administer Upstaza:
- The target infusion points are defined according to the reference stereotactic neurosurgical practice. Upstaza is administered as bilateral infusion (2 infusions per putamen) with an intracranial cannula. The 4 final points of each trajectory should be defined as 2 mm in the posterior (above) direction from the anterior and posterior target points in the mid-horizontal plane (Figure 1).
Figure 1 Four desired locations for injection points
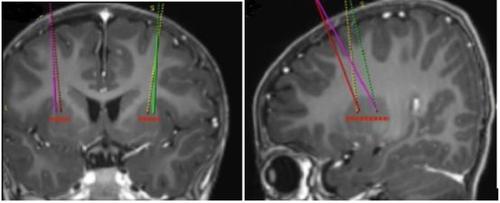
- Once the stereotactic registration is complete, the entry point on the skull should be marked. Surgical access should be performed through the skull bone and dura mater.
- The infusion cannula is placed at the designated point in the putamen using stereotactic means from the planned trajectories. Note that the infusion cannula is placed and infusion is performed separately for each putamen.
- Upstaza is injected at a rate of 0.003 ml/min at each of the 2 target points in each putamen; 0.08 ml of Upstaza is injected per putamen point, resulting in 4 infusions with a total volume of 0.320 ml (or 1.8 × 10^11 vg).
- Starting with the first target point, the cannula is inserted through a trephine hole in the putamen and then slowly withdrawn, distributing the 0.08 ml of Upstaza through the planned trajectory to optimize distribution in the putamen.
- After the first infusion, the cannula is removed and reinserted at the next target point, repeating the same procedure for the other 3 target points (anterior and posterior of each putamen).
- After standard neurosurgical closure procedures, the patient undergoes a postoperative computed tomography scan to check for postoperative complications (e.g., hemorrhages).
- The patient should be accommodated near the hospital where the intervention was performed for at least the first 48 hours after the intervention. The patient may return home after the intervention, according to the discretion of the treating physician. Post-treatment care should be directed by the reference pediatric neurologist and neurosurgeon. The patient will be followed up 7 days after the intervention to check for complications. A second follow-up visit will take place 3 weeks after the intervention to monitor post-surgical recovery and the presence of adverse events.
- Patient will be offered the possibility to enroll in a registry to further evaluate the long-term safety and efficacy of the treatment under normal clinical practice conditions.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to UPSTAZA 2.8 × 10^11 GENE THERAPY VECTORS (VG)/0.5 ML SOLUTION FOR INFUSIONDosage form: INJECTABLE INFUSION, 100 UActive substance: laronidaseManufacturer: Sanofi B.V.Prescription requiredDosage form: INJECTABLE PERFUSION, 30 mg/mlActive substance: cerliponase alfaManufacturer: Biomarin International LimitedPrescription requiredDosage form: INJECTABLE INFUSION, UnknownActive substance: imigluceraseManufacturer: Sanofi B.V.Prescription required
Online doctors for UPSTAZA 2.8 × 10^11 GENE THERAPY VECTORS (VG)/0.5 ML SOLUTION FOR INFUSION
Discuss questions about UPSTAZA 2.8 × 10^11 GENE THERAPY VECTORS (VG)/0.5 ML SOLUTION FOR INFUSION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















