TRIXEO AEROSPHERE 5 mcg/7.2 mcg/160 mcg PRESSURED INHALATION SUSPENSION


How to use TRIXEO AEROSPHERE 5 mcg/7.2 mcg/160 mcg PRESSURED INHALATION SUSPENSION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Trixeo Aerosphere 5 micrograms/7.2 micrograms/160 micrograms inhalation suspension in a pressurised container
formoterol fumarate dihydrate/glycopyrronium/budesonide
Read this package leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this package leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this package leaflet. See section 4.
Contents of the package leaflet
- What is Trixeo Aerosphere and what is it used for
- What you need to know before you start using Trixeo Aerosphere
- How to use Trixeo Aerosphere
- Possible side effects
- Storage of Trixeo Aerosphere
- Contents of the pack and further information
- Instructions for use
1. What is Trixeo Aerosphere and what is it used for
Trixeo Aerosphere contains three active substances called formoterol fumarate dihydrate, glycopyrronium, and budesonide.
- Formoterol fumarate dihydrate and glycopyrronium belong to a group of medicines called “bronchodilators”. They work by blocking the constriction of the muscle in the airways to make it easier to breathe in and out.
- Budesonide belongs to a group of medicines called “corticosteroids”, which work by reducing inflammation in the lungs.
Trixeo Aerosphere is an inhaler used in adults with a lung disease called “chronic obstructive pulmonary disease” (or “COPD”), a long-term disease of the airways in the lungs.
Trixeo Aerosphere is used to make breathing easier and to improve the symptoms of COPD such as shortness of breath, wheezing, and coughing. Trixeo Aerosphere can also prevent COPD attacks (exacerbations).
When inhaled, Trixeo Aerosphere releases the active substances in the lungs. If you use this medicine regularly, twice a day, it will help reduce the effects of COPD on your daily life.
2. What you need to know before you start using Trixeo Aerosphere
Do not use Trixeo Aerosphere
- if you are allergic to formoterol fumarate dihydrate, glycopyrronium, budesonide, or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Trixeo Aerosphere is used as a long-term maintenance treatment for COPD. Do not use it to treat a sudden attack of shortness of breath or wheezing.
Immediate breathing difficulties
If you notice chest tightness, coughing, wheezing, or difficulty breathing immediately after using Trixeo Aerosphere, stop using it and tell your doctor immediately(see “Serious side effects” at the beginning of section 4 for more information).
If your difficulty breathing, chest tightness, wheezing, or coughing gets worse during treatment with Trixeo Aerosphere, you should continue to use Trixeo Aerosphere but contact your doctor as soon as possible, as you may need additional treatment.
Tell your doctor before using Trixeo Aerosphere if:
- you have high blood pressure or heart problems
- you are diabetic
- you have a lung infection
- you have thyroid gland problems
- you have low potassium levels in your blood
- you have prostate problems or difficulty urinating
- you have a certain eye problem called “narrow-angle glaucoma”
- you have kidney or liver problems
Tell your doctor if you think any of these apply to you.
Children and adolescents
Trixeo Aerosphere has not been studied in children and adolescents. Do not give this medicine to children or adolescents under 18 years of age.
Other medicines and Trixeo Aerosphere
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicines. This includes medicines obtained without a prescription and herbal products. This is because Trixeo Aerosphere may affect how other medicines work. Also, some medicines may affect how Trixeo Aerosphere works or increase the chance of you having side effects.
Tell your doctor or pharmacist if you are taking any of the following medicines:
- beta-blocker medicines (such as atenolol or propranolol), which may be used to treat high blood pressure or heart problems, or to treat glaucoma (such as timolol)
- medicines used to treat fungal infections, such as ketoconazole or itraconazole
- medicines used to treat HIV infection, such as ritonavir or cobicistat
- medicines that lower the amount of potassium in the blood, such as:
- corticosteroids taken by mouth (such as prednisolone)
- diuretics - medicines that increase urine production (such as furosemide or hydrochlorothiazide), which may be used to treat high blood pressure
- certain medicines used to treat breathing problems (such as theophylline), called “methylxanthines”
- any medicine that works in the same way as Trixeo Aerosphere, such as tiotropium, ipratropium, aclidinium, umeclidinium, or salmeterol, arformoterol, vilanterol, olodaterol, or indacaterol. Do not use Trixeo Aerosphere if you are already using these medicines.
- medicines used to treat heart rhythm problems, such as amiodarone
- medicines that may partly affect the heart’s electrical activity (called “QT interval”), such as:
- medicines for depression (such as monoamine oxidase inhibitors or tricyclic antidepressants),
- medicines for bacterial infections (such as erythromycin, clarithromycin, or telithromycin),
- medicines for allergic reactions (antihistamines).
If you are in any of these situations, or if you are unsure, talk to your doctor or pharmacist before using Trixeo Aerosphere.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctor or pharmacist for advice before using this medicine.
Do not use Trixeo Aerosphere if you are pregnant unless your doctor tells you you can.
Do not use this medicine if you are breastfeeding unless your doctor tells you you can.
Driving and using machines
It is unlikely that this medicine will affect your ability to drive or use machines. However, dizziness is an uncommon side effect that you should be aware of when driving or using machines.
3. How to use Trixeo Aerosphere
Follow the instructions for using this medicine exactly as your doctor has told you.
If you are unsure, ask your doctor or pharmacist again.
How much to use
The recommended dose is two inhalations, twice a day - two inhalations in the morning and two inhalations in the evening.
It is important that you use Trixeo Aerosphere every day, even if you do not have symptoms of COPD at that time.
Remember:Always rinse your mouth with water after inhaling Trixeo Aerosphere. This helps remove any remaining medicine from your mouth. After rinsing, spit out the water; do not swallow it.
How to use
Trixeo Aerosphere is for inhalation use only.
Read the “Instructions for use” at the end of this package leaflet. If you are not sure how to use Trixeo Aerosphere, ask your doctor or pharmacist.
Using Trixeo Aerosphere with a spacer
You may find it difficult to breathe in and press the inhaler at the same time. If this happens, ask your doctor or pharmacist for advice. Using a “spacer” with the inhaler may be helpful.
If you use more Trixeo Aerosphere than you should
If you have used more Trixeo Aerosphere than you should, talk to your doctor or pharmacist immediately. You may need medical attention. You may notice that your heart is beating faster than usual or that you have tremors, changes in vision, dry mouth, headache, or nausea.
If you forget to use Trixeo Aerosphere
Do not take a double dose to make up for a forgotten dose. Use it as soon as you remember.
However, if it is almost time for your next dose, skip the missed dose. Do not use more than two inhalations, twice a day, on the same day.
If you stop using Trixeo Aerosphere
This medicine is for long-term use. Use this medicine for as long as your doctor tells you. It will only work if you use it.
Do not stop using it unless your doctor tells you to, even if you feel better, as your symptoms may get worse. If you want to stop treatment, talk to your doctor first.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them. The following side effects may happen with this medicine:
Serious side effects
Uncommon(may affect up to 1 in 100 people)
Immediate breathing difficulties:
- If you get difficulty breathing immediately after using Trixeo Aerosphere, such as chest tightness, coughing, wheezing, or shortness of breath, stop using this medicineand tell your doctor immediately.
Allergic reactions:
- swelling of the face, especially around the mouth (swelling of the tongue or throat may cause difficulty swallowing)
- skin rash or hives with difficulty breathing
- a sudden feeling that you are going to faint
These symptoms may be signs of a serious allergic reaction. Stop using this medicine and seek medical help immediately if you notice any of these serious side effects.
Other side effects
Tell your doctor or pharmacist if you notice any of the following side effects:
Common(may affect up to 1 in 10 people)
- oral thrush (a fungal infection). This can be prevented by rinsing your mouth with water after inhaling Trixeo Aerosphere.
- anxiety
- difficulty sleeping
- nausea
- headache
- coughing or hoarseness
- muscle cramps
- awareness of heartbeats (palpitations)
- high blood sugar levels (detected by blood tests)
- pain when urinating and increased frequency of urination (may be signs of a urinary tract infection)
- pneumonia (lung infection).
Tell your doctor if you get any of the following symptoms while using Trixeo Aerosphere, as they may be signs of a lung infection:
- fever or chills,
- increased production of mucus, change in mucus color,
- increased coughing or difficulty breathing.
Uncommon(may affect up to 1 in 100 people)
- tremors or feeling dizzy
- dry mouth or mild throat irritation
- bruising on the skin
- feeling restless, nervous, or agitated
- depression
- fast or irregular heartbeats
- chest pain or tightness (angina)
Very rare(may affect up to 1 in 10,000 people)
- changes in behavior
- effects on the adrenal glands
Frequency not known(frequency cannot be estimated from the available data):
- blurred vision
- clouding of the lens in the eye (signs of cataract)
- increased pressure in the eyes (glaucoma)
- swelling of the face, especially around the mouth (swelling of the tongue or throat may cause difficulty swallowing)
Reporting of side effects
If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this package leaflet. You can also report side effects directly via the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Trixeo Aerosphere
Keep this medicine out of the sight and reach of children.
Do not use Trixeo Aerosphere after the expiry date which is stated on the carton, pouch, and pressurised container after EXP. The expiry date is the last day of the month stated.
Once the pouch is opened, the inhaler must be used within 3 months.
Keep the inhaler in the pouch; only open the pouch to remove the inhaler immediately before first use. On the day you open the pouch, write the date on the inhaler label in the space provided.
Do not store above 30°C. Store in a dry place.
For best results, the inhaler should be at room temperature before use.
Do not break, pierce, or burn the pressurised container, even when it appears to be empty. Do not use it or store it near a heat source or open flame.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Container Contents and Additional Information
Composition of Trixeo Aerosphere
The active ingredients are formoterol fumarate dihydrate, glycopyrronium, and budesonide.
Each individual inhalation provides a delivered dose (the dose that comes out of the mouthpiece) of 5 micrograms of formoterol fumarate dihydrate, 9 micrograms of glycopyrronium bromide equivalent to 7.2 micrograms of glycopyrronium, and 160 micrograms of budesonide.
The other components are norflurane, 1,2-distearyl-sn-glycero-3-phosphocholine, and calcium chloride.
This medication contains fluorinated greenhouse gases. Each inhaler contains 10.6 g of norflurane (HFC-134a) which corresponds to 0.015 tons of CO2 equivalent (global warming potential GWP = 1,430).
Appearance of the Product and Container Contents
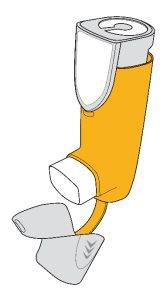
Trixeo Aerosphere is an inhalation suspension in a pressurized container.
Trixeo Aerosphere is presented in a cartridge with a dose indicator, provided with a yellow plastic adapter and a white mouthpiece. The mouthpiece is covered by a gray removable protective cap.
Trixeo Aerosphere is supplied in an aluminum bag containing a desiccant sachet, and packaged in a cardboard box.
Each inhaler contains 120 sprays. Additionally, there are multiple packs containing 3 pressurized inhalers with 120 inhalations each.
Marketing Authorization Holder
AstraZeneca AB
SE-151 85 Södertälje
Sweden
Manufacturer
AstraZeneca Dunkerque Production
224 Avenue de la Dordogne
Dunkerque
59640
France
You can request more information about this medication by contacting the local representative of the marketing authorization holder:
België/Belgique/Belgien AstraZeneca S.A./N.V. Tel: +32 2 370 48 11 | Lietuva UAB AstraZeneca Lietuva Tel: +370 5 2660550 |
| Luxembourg/Luxemburg AstraZeneca S.A./N.V. Tél/Tel: +32 2 370 48 11 |
Ceská republika AstraZeneca Czech Republic s.r.o. Tel: +420 222 807 111 | Magyarország AstraZeneca Kft. Tel.: +36 1 883 6500 |
Danmark AstraZeneca A/S Tlf: +45 43 66 64 62 | Malta Associated Drug Co. Ltd Tel: +356 2277 8000 |
Deutschland AstraZeneca GmbH Tel: +49 40 809034100 | Nederland AstraZeneca BV Tel: +31 85 808 9900 |
Eesti AstraZeneca Tel: +372 6549 600 | Norge AstraZeneca AS Tlf: +47 21 00 64 00 |
Ελλάδα AstraZeneca A.E. Τηλ: +30 210 6871500 | Österreich AstraZeneca Österreich GmbH Tel: +43 1 711 31 0 |
España AstraZeneca Farmacéutica Spain, S.A. Tel: +34 91 301 91 00 | Polska AstraZeneca Pharma Poland Sp. z o.o. Tel.: +48 22 245 73 00 |
Francia AstraZeneca Tél: +33 1 41 29 40 00 | Portugal AstraZeneca Produtos Farmacêuticos, Lda. Tel: +351 21 434 61 00 |
Hrvatska AstraZeneca d.o.o. Tel: +385 1 4628 000 | România AstraZeneca Pharma SRL Tel: +40 21 317 60 41 |
Ireland AstraZeneca Pharmaceuticals (Ireland) DAC Tel: +353 1609 7100 | Slovenija AstraZeneca UK Limited Tel: +386 1 51 35 600 |
Ísland Vistor hf. Sími: +354 535 7000 | Slovenská republika AstraZeneca AB, o.z. Tel: +421 2 5737 7777 |
Italia AstraZeneca S.p.A. Tel: +39 02 00704500 | Suomi/Finland AstraZeneca Oy Puh/Tel: +358 10 23 010 |
Κύπρος Αλκήτωρ Φαρμακευτική Λτδ Τηλ: +357 22490305 | Sverige AstraZeneca AB Tel: +46 8 553 26 000 |
Latvija SIA AstraZeneca Latvija Tel: +371 67377100 | United Kingdom (Northern Ireland) AstraZeneca UK Ltd Tel: +44 1582 836 836 |
Date of Last Revision of this Leaflet:
Detailed information on this medicinal product is available on the European Medicines Agency website: http://www.ema.europa.eu.
---------------------------------------------------------------------------------------------------------------------------
Read Before Using the Inhaler
INSTRUCTIONS FOR USE
TRIXEO AEROSPHERE
(formoterol fumarate dihydrate, glycopyrronium, and budesonide)
Inhalation suspension in a pressurized container
For oral inhalation use only
Please read these instructions carefully.
Trixeo Aerosphere (referred to as “inhaler” in this leaflet) may be different from other inhalers you have used before.
Important Information
- For oral inhalation use only
- Prime your inhaler before first use by priming
- Wash the yellow adapter weekly
- Take 2 inhalations of the medication in the morning and 2 inhalations of the medication in the evening
Storing Your Inhaler
- Do not store above 30°C. Store in a dry place
- Do not store in a humid environment, such as a bathroom
- Keep your inhaler and all medications out of sight and reach of children
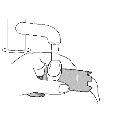
Parts of Your Inhaler
| Dose IndicatorIncorporated into the top of the pressurized cartridge. |
Pressurized Cartridge (inside)Contains the medication. | |
AdapterContains the pressurized cartridge. | |
MouthpieceSprays the medication. | |
Protective CapProtects the mouthpiece when the inhaler is not in use. |
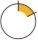
Reading the Dose Indicator
Each time you take an inhalation of the medication, the dose indicator will reduce by one unit the number of doses indicated. | |
ArrowIndicates the number of doses remaining |
|
Yellow ZoneOrder a new inhaler when the arrow is in the yellow zone | |
Red ZoneDiscard your inhaler when the arrow marks 0 in the red zone | |
Do not attempt to take an inhalation when the arrow indicates 0 because you will not receive the full dose. |
Order a New Inhaler
- Order a new inhaler when the arrow on the dose indicator is in the yellow zone.
Discard Your Inhaler
Discard your inhaler following local guidelines when:
- the dose indicator marks 0
or
- 3 monthshave passed after removing the inhaler from the aluminum bag
Do not reuse the adapter or use it with cartridges containing medication from other inhalers.
Do not puncture the cartridge or throw it into a fire or incinerator.
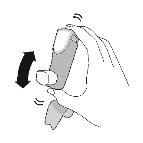
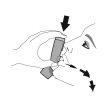
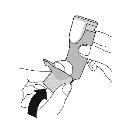
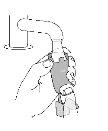
BEFORE FIRST USE – Prime your inhaler 4 times before first use
- Before using your inhaler for the first time, prepare it to receive the correct amount of medication when you use it.
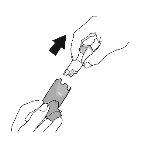
Priming. Step 1 |
Remove the protective cap from the mouthpiece. |
|
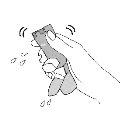
Priming. Step 2 | |||
Shake the inhaler well and take 1 priming sprayinto the air without aiming it at yourself. Repeat until a total of 4 priming spraysare reached, shaking before each priming spray. | |||
|
|
| |
Extra doses are provided for priming. Do not skip priming. |
Re-prime your inhaler:
| To re-prime, take 2 priming sprays, shaking before each priming spray.
|
DAILY USE, morning & evening – Inhale your medication
- Daily dose: 2 inhalations in the morning and 2 inhalations in the evening.
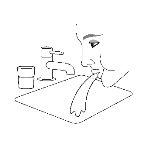
- Rinse your mouth with water after the 2 inhalations to prevent fungal infections.
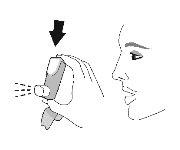
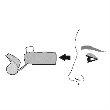
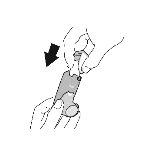
Use. Step 1 |
Remove the protective cap from the mouthpiece. Check that there are no foreign objects in the mouthpiece and remove any object before use. |
|
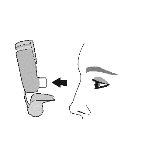
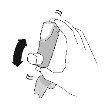
Use. Step 2 | |||||
Shake the inhaler well before each inhalation. | Expel all the air from your mouth. | Place the mouthpiece in your mouth and close your lips around the mouthpiece. Tilt your head back, keeping your tongue below the mouthpiece. | Start taking a slow and deep breath in while spraying 1dose.Continue inhaling until you can no longer inhale. | Hold your breath for as long as you can for a maximum of 10seconds. | |
|
|
|
|
|
|
Use. Step 3 | Use. Step 4 | Use. Step 5 |
| Replace the protective cap on the mouthpiece. | Rinse your mouth with water. Spit out the water. Do not swallow. |
|
|
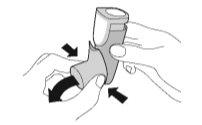
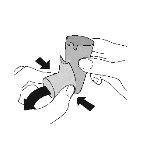
WEEKLY CLEANING – Wash your adapter once a week
- Wash the yellow adapter weeklyso that the medication does not accumulate and block the spray through the mouthpiece.
- Do not get the cartridge wet.
- Re-prime after cleaning.
Cleaning. Step 1 | Cleaning. Step 2 |
Remove the cartridge and set it aside. Do not get the cartridge wet. | Remove the protective cap from the mouthpiece. |
|
|
Cleaning. Step 3 | Cleaning. Step 4 | ||||
Run warm water through the mouthpiece for 30 seconds and then through the top of the adapter for 30 seconds. Wash for 60 seconds in total. | Shake off as much water as you can. | ||||
|
|
|
|
|
|
Cleaning. Step 5 | Cleaning. Step 6 | |
Inspect the inside of the adapter and mouthpiece for any accumulation of medication. If there is any accumulation, repeat Cleaning Steps 3 to 5. | Allow to air dry, preferably overnight. Do notreinsert the cartridge into the adapter if the adapter is still wet. | |
|
|
|
Cleaning. Step 7 | Cleaning. Step 8 |
Once dry, first replace the mouthpiece capand then gently press the cartridge down into the adapter. | Re-prime the inhaler by taking 2 priming sprays, shaking before each priming spray. |
|
|
- Country of registration
- Average pharmacy price72.59 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to TRIXEO AEROSPHERE 5 mcg/7.2 mcg/160 mcg PRESSURED INHALATION SUSPENSIONDosage form: PULMONARY INHALATION, 55 MICROGRAMS/22 MICROGRAMSActive substance: vilanterol and umeclidinium bromideManufacturer: Glaxosmithkline (Ireland) LimitedPrescription requiredDosage form: PULMONARY INHALATION, 340/12 microgramsActive substance: formoterol and aclidinium bromideManufacturer: Covis Pharma Europe B.V.Prescription requiredDosage form: PULMONARY INHALATION, 0.5 mg/2.5 mgActive substance: salbutamol and ipratropium bromideManufacturer: Genetic S.P.A.Prescription required
Online doctors for TRIXEO AEROSPHERE 5 mcg/7.2 mcg/160 mcg PRESSURED INHALATION SUSPENSION
Discuss questions about TRIXEO AEROSPHERE 5 mcg/7.2 mcg/160 mcg PRESSURED INHALATION SUSPENSION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions