
TRANILCIPROMINE ARISTO 20 mg FILM-COATED TABLETS

How to use TRANILCIPROMINE ARISTO 20 mg FILM-COATED TABLETS
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Tranilcipromina Aristo 20 mg Film-Coated Tablets
Read this package leaflet carefully before you start taking this medicine, because it contains important information for you.
- Keep this package leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this package leaflet. See section 4.
Contents of the Package Leaflet
- What is Tranilcipromina Aristo and what is it used for
- What you need to know before you start taking Tranilcipromina Aristo
- How to take Tranilcipromina Aristo
- Possible side effects
- Storing Tranilcipromina Aristo
- Package Contents and Additional Information
1. What is Tranilcipromina Aristo and what is it used for
This medicine contains the active substance tranilcipromina, which belongs to the group of monoamine oxidase inhibitors (MAOIs).
Tranilcipromina is used for the treatment of depressive disorders (episodes of major depressive disorder) in adults (from 18 years of age).
Tranilcipromina should be used when other medicines for depression have not produced a satisfactory improvement or cannot be used, i.e., as a so-called reserve antidepressant.
2. What you need to know before starting to take Tranilcipromina Aristo
Do not taketranilcipromina
Do not take tranilcipromina if you are taking one of the following medicines at the same time:
- Citalopram, escitalopram, fluoxetine, fluvoxamine, paroxetine, sertraline, and other so-called "selective serotonin reuptake inhibitors" (a group of agents used to treat depression). There is a risk of triggering the so-called serotonin syndrome with symptoms such as involuntary and rhythmic muscle contractions, including the muscles that control eye movement, agitation, increased blood pressure, hallucinations, coma, excessive sweating, tremors, exaggerated reflexes, increased muscle tension, irritability, and increased body temperature above 38°C.
- Vortioxetine, an agent for the treatment of depression. There is a risk of serotonin syndrome (see first item).
- Venlafaxine, duloxetine, and milnacipran, agents for the treatment of depression. There is a risk of serotonin syndrome (see first item).
- Sibutramine, an agent for the treatment of overweight (which is no longer used today). There is a risk of serotonin syndrome (see first item).
- Clomipramine, an agent used to treat depression. There is a risk of serotonin syndrome (see first item).
- Sumatriptan, zolmitriptan, naratriptan, rizatriptan, eletriptan, and other so-called "triptans", agents used to treat migraine. There is a risk of serotonin syndrome (see first item).
- L-tryptophan. Delirium symptoms may appear.
- Buspirone, an agent for the treatment of anxiety and agitation. A strong increase in blood pressure has been reported.
- Imipramine, an agent used to treat depression. Serious adverse effects such as irritability, coma, increased body temperature, convulsions, and large fluctuations in blood pressure, especially increased blood pressure, may occur.
- Certain agents to increase blood pressure, but which may also be contained in nasal remedies, cough suppressants, or anti-cold and appetite suppressants (so-called indirect sympathomimetics, e.g., ephedrine, amezinium methyl sulfate, phenylpropanolamine, cathine, amfepramone, metamfepramone, and amphetamines, also known as "stimulant amines"). There is a risk of severe hypertensive crises, characterized by a sudden increase in blood pressure.
- Pethidine (agent for severe pain), tramadol (agent for moderate pain), and dextromethorphan (contained in cough suppressants). Life-threatening adverse effects may occur in the central nervous system or life-threatening influences on respiratory and circulatory functions.
- Disulfiram, a deterrent agent for alcohol consumption. Delirium is possible.
- Levodopa (agent for the treatment of Parkinson's disease), provided it is not combined with agents such as benserazide or carbidopa. There is a risk of uncontrolled increase in blood pressure.
Warnings and precautions
Consult your doctor or pharmacist before starting to take this medicine.
Special caution is required when taking this medicine,
- with food and drink. You should not consume foods, stimulants, or drinks with high levels of tyramine during a period of 1 day before treatment until 14 days after treatment with tranilcipromina, which is also known as a low-tyramine diet (see section 2, "Taking tranilcipromina with food, drinks, and alcohol"). You should also not drink any alcohol during treatment with tranilcipromina. Alcohol changes and increases the effect of tranilcipromina in an unpredictable way;
Suicidal thoughts and worsening of depression
If you are depressed, you may sometimes have thoughts of harming or killing yourself (suicide). These thoughts can be stronger when you first start using antidepressants, as all these medicines take time to work, usually a couple of weeks, sometimes more.
It is more likely that such thoughts will occur
If at any time you have thoughts of harming or killing yourself, consult your doctor or go to the hospital immediately.
It may be helpful if you tell a friend or relative that you are depressed. Ask them to read this leaflet. Ask them to tell you if they think your depression is getting worse or if they are worried about changes in your behavior.
Older patients
If you are over 65 years old, your doctor will slowly increase the daily dose, keep the dose as low as possible, and monitor your blood pressure regularly (see section 3, "Dose in older patients").
Children and adolescents
Tranilcipromina should not be used in children and adolescents.
Other medicines and Tranilcipromina
Tell your doctor or pharmacist if you are taking, have recently taken, or might take any other medicines.
Tranilcipromina should not be taken at the same time as certain medicines. Read section 2, "What you need to know before starting to take Tranilcipromina Aristo", carefully, especially the section "Do not take Tranilcipromina if you are taking one of the following medicines at the same time", and consult your doctor.
Also, notethat certain medicinesthat are incompatiblewith tranilcipromina require a treatment-free period. If you switchfrom another medicine to tranilcipromina, the treatment-free periodwith the previously used medicine is based on the time it takes for the active ingredient to be eliminated from the body. If you have recently finished a treatment with tranilcipromina, you must wait at least 14 days before you can start taking another medicine.
Increased risk of adverse effects of tranilcipromina
Avoid taking tranilcipromina at the same time as certain so-called direct sympathomimetics (e.g., those contained in medicines for circulatory problems, to relax bronchial muscles, or in nasal drops).
The combination with selective β2-sympathomimetics for inhalation is not associated with any particular risk.
If you suffer from persistent depression that has been treated with other medicines for the treatment of depression (tricyclic antidepressants, e.g., amitriptyline) without satisfactory improvement, your doctor may, in individual cases, administer an additional dose of tranilcipromina with a slow increase in the dose. However, this does not apply to clomipramine, imipramine, or antidepressants administered by infusion.
Tranilcipromina may increase the effect of other medicines and even increase the risk of adverse effects
The blood pressure-lowering effect of agents for hypertension (e.g., guanethidine, methyldopa) may be potentiated with tranilcipromina. However, in individual cases, an increase in blood pressure may also be triggered by states of excitement.
The effect of insulinand oral agents (agents that must be taken orally) against diabetes may be enhanced (see section 2, "Warnings and precautions").
The adverse effects of bupropionor anfebutamone(agent for smoking cessation), such as convulsions and states of excitement, may be intensified when taking tranilcipromina at the same time. Therefore, avoid this combination.
The effect of neuroleptics, antidepressants, benzodiazepines, and analgesics(medicines with a calming effect on the brain) may be potentiated when taking tranilcipromina at the same time.
In rare cases, the occurrence of serotonin syndrome has been reported with certain agents for the treatment of depression known to cause potentially fatal serotonin syndrome and simultaneous treatment with buprenorphine (agent for severe pain). It cannot be ruled out that serotonin syndrome may also occur with simultaneous treatment of buprenorphineand tranilcipromina. The symptoms of serotonin syndrome are described in section 2 under the heading "Do not take tranilcipromina if you are taking one of the following medicines at the same time". Inform your doctor if you experience symptoms of serotonin syndrome.
Interactions during surgery and dental treatment
If you are scheduled to undergo surgery with anestheticsand certain analgesics, the doctor must discontinue the administration of tranilcipromina 14 days before the operation. Interactions between medicines similar to tranilcipromina and anesthetics have been reported, which in some cases have been severe (e.g., unstable circulation, coma).
Please inform the anesthesiologist that you are taking this medicine before surgery. Pethidine, a strong analgesic used, for example, for postoperative pain therapy, should never be administered when being treated with tranilcipromina.
Inhalation anesthetics(volatile agents used to produce anesthesia) do not pose a greater risk for patients treated with this medicine than for any other patient under anesthesia. Ether is the only exception and should not be used.
Local anesthetics:The generally low concentrations of adrenaline or noradrenaline in agents for local anesthesia, for example, in dental interventions or eye drops, do not pose a special risk for patients treated with tranilcipromina.
Note that this information may also apply to recently used medicines.
Taking tranilcipromina with food, drinks, and alcohol
When taking tranilcipromina, you should pay attention to the problemof biogenic amines(especially tyramine). Biogenic amines are natural ingredients in many foods. In most cases, their content in unprocessed animal and plant foods is very low. In contrast, certain processing methods involving microbiological transformations (e.g., fermentation in cheese production) can significantly increase the content of biogenic amines, but also as a result of storage at too high a temperature or spoilage.
In low concentrations, biogenic amines are not hazardous to humans, as the human body has enzymes (mono- and diamine oxidases) that break down the amines. However, the ingestion of higher concentrations of biogenic amines can produce a toxic (poisonous) effect, especially if medicines like tranilcipromina with an amine oxidase-blocking effect are taken at the same time. The toxic effect of biogenic amines can manifest as nausea, vomiting, occipital headaches, and diseases of the nervous system, but above all as changes in blood pressure up to a marked increase in blood pressure (hypertensive crisis).
From one day before treatment with tranilcipromina, during treatment, and up to 14 days after the last dose, you should avoid certain foods and drinks with a relatively high content of biogenic amines (e.g., air-dried, fermented, or aged meat, sausages, salami, fish or poultry, aged cheese, beans, fish sauces, all fermented soy products, yeast extracts, fermented beverages like beer or wine). This low-tyramine diet applies to all doses of tranilcipromina.
Please use all foodsas freshas possible. Consume raw or partially cooked foods on the day of preparation. Use opened semi-preserved foods and thawed frozen foods immediately. You can store opened preserves or fully cooked foods in the refrigerator for a maximum of 48 hours until consumption. Set your refrigerator to a low temperature of <4 °c if possible.< p>
At the end of the leaflet, you will find a list of allowed, prohibited, and allowed in small quantitiesfoods (according to their content of biogenic amines). Please note: only one food per meal is recommended, which is allowed in small quantities.
You should also not drink any alcoholduring treatment with this medicine. Alcohol changes and increases the effect of tranilcipromina in an unpredictable way.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medicine.
Pregnancy
There is not enough experience with the use of tranilcipromina in pregnant women. This medicine may have negative effects on the fetus. Existing high blood pressure in the mother may be exacerbated, and a reduction in blood circulation in the placenta is possible.
Therefore, your doctor will only prescribe this medicine during pregnancy, especially in the first trimester of pregnancy, if it is absolutely necessary. Tell your doctor immediately if you are planning to become pregnant or if you suspect you are pregnant. Your doctor may then switch to another medicine if necessary.
Breastfeeding
You should not take tranilcipromina during breastfeeding. If your doctor considers it necessary to use it during breastfeeding, you must stop breastfeeding.
Driving and using machines
Tranilcipromina has little or moderate influence on the ability to drive and use machines. Therefore, even when used as intended, and especially in the first few days of treatment, tranilcipromina may affect the ability to actively participate in road traffic and use machines. This is particularly true in combination with substances that act on the central nervous system. Remember that you should not drink alcohol during treatment with tranilcipromina!
During the first daysof treatment, you must not drivea caror other vehicles, nor use machines or electrical appliances, nor perform work that requires a lot of attention, e.g., those that are performed without a firm grip.
The decision will be made by your treating doctor in each case, taking into account your individual reaction and the dose.
Tranilcipromina contains lactose
This medicine contains lactose. If your doctor has told you that you have an intolerance to certain sugars, consult with them before taking this medicine.
3. How to take Tranilcipromina Aristo
Follow the administration instructions for this medication exactly as indicated by your doctor or pharmacist. In case of doubt, consult your doctor or pharmacist again.
Unless the doctor prescribes otherwise, the usual dose is:
At the beginning of treatment, take ½ tablet of tranilcipromine 20 mg once a day in the morning. Your doctor may increase this dose by 10 mg of tranilcipromine (corresponding to ½ film-coated tablet of Tranilcipromina 20 mg) per week until the adequate total daily dose for you is reached.
The recommended total daily dose is 20 mg to 40 mg of tranilcipromine (equivalent to 1 to 2 film-coated tablets of Tranilcipromina 20 mg).
Normally, the mood-elevating effects and relief from depression can be expected to occur after 1 to 3 weeks. Your doctor will check the dose during therapy and adjust it if necessary.
If the response of the disease to the recommended total daily dose of 2 to 4 film-coated tablets of [product name] is not satisfactory, the doctor may increase the dose in steps of ½ film-coated tablet per day every 1 to 3 weeks, doing so under inpatient care. The maximum total daily dose is 3 film-coated tablets of tranilcipromine.
Once the depressive disorder has improved, your doctor may reduce the total daily dose to a maintenance dose of ½ to 1 film-coated tablet of Tranilcipromina 20 mg.
Dose in elderly patients (over 65 years old)
In the case of patients over 65 years old, the attending doctor will increase the dose more slowly under regular blood pressure control.
Patients with renal insufficiency
Patients with severe renal insufficiency should not be treated with this medication, as there is not enough therapeutic experience. If you have impaired renal function, your doctor will carefully monitor the treatment progress (see section 2. "Warnings and precautions").
Patients with hepatic insufficiency
Tranilcipromine should not be used to treat patients with hepatic insufficiency (see section 2. "Do not take tranilcipromine").
Use in children and adolescents
Tranilcipromine should not be used in children and adolescents (see section 2. "Do not take tranilcipromine").
Method of administration
The film-coated tablets are taken orally.
Take this medication exactly as your doctor has indicated. If you have doubts, consult your doctor or pharmacist.
Take the film-coated tablets with sufficient liquid, preferably a glass of water, and do not chew them.
The tablet can be divided into equal doses.
The total daily dose can be divided into 1 to 3 times. Do not take the last film-coated tablet of tranilcipromine later than 3 pm to avoid sleep disturbance.
Indications for facilitating division
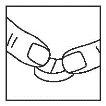
Place the tablet on a firm and flat surface (with the notch facing up). Use your thumbs or index fingers to press the tablet from above, on both sides of the notch, to divide it into two parts.
Duration of treatment
The duration of a treatment period with tranilcipromine until the improvement of a depressive disease is usually at least 4 to 6 weeks. Your doctor may continue the treatment with tranilcipromine at a reduced dose for 4 to 6 months.
If you switch from another agent for the treatment of depression to tranilcipromine, your doctor will generally prescribe a treatment-free period of at least 7 days and will only prescribe 1 film-coated tablet of tranilcipromine 20 mg for at least the first week after starting treatment.
It is important not to stop treatment or change the dose without consulting your doctor.
Please consult your doctor or pharmacist if you consider that the effect of this medication is too strong or too weak.
If you take more Tranilcipromina than you should
Inform your doctor immediatelyso that they can decide what measureto take.
The signs of overdoseinclude confusion, overexcitement up to cerebral convulsive crises, obnubilation up to fainting, fever, deterioration of respiratory function (up to respiratory arrest) and of the cardiovascular system (intense fluctuations in blood pressure, irregular heartbeats) as well as of the muscles (strong muscle spasms).
In certain circumstances, the signs of an overdose may not appear until several hours after taking the tablets.
In case of overdose or accidental ingestion, consult your doctor or pharmacist or call the Toxicology Information Service, phone 91 562 04 20, indicating the medication and the amount ingested.
If you forget to take tranilcipromina
Do not take a double dose to make up for forgotten doses. Continue taking the dose at the next scheduled time as prescribed.
If you interrupt treatment with tranilcipromina
Withdrawal symptoms such as anxiety, restlessness, sleep disturbances, stupor, or delirium may appear. Avoid suddenly interrupting long-term high-dose therapy with tranilcipromina. Always end therapy under medical supervision by gradually reducing the dose. Inform your doctor if you experience such withdrawal symptoms after reducing the dose or after stopping this medication. You may need to take the last dose again and then reduce the dose in smaller intervals.
If you have any doubts about taking this medication, consult your doctor or pharmacist.
4. Possible adverse effects
Like all medications, this medication can cause adverse effects, although not all people experience them.
If you experience an unusually elevated or irritable mood (manic disorder), do not continue taking this medication and consult your doctor as soon as possible.
Other adverse effects
The following adverse effects are very common, especially at the beginning of treatment: sleep disturbances, low blood pressure, decrease in blood pressure when standing up.
Very common:may affect more than 1 in 10 people:
- insomnia, sleep disturbances;
- decrease in blood pressure when standing up, low blood pressure.
Common:may affect up to 1 in 10 people:
- anxiety, hyperactivity, agitation;
- dizziness, dry mouth, fatigue;
- palpitations;
- high blood pressure (arterial hypertension);
- weight gain, weight loss, weakness.
Uncommon:may affect up to 1 in 100 people:
- severe increases in blood pressure (hypertensive crises), which may be accompanied by rapid heartbeats or palpitations, facial flushing, headaches (especially occipital headaches), neck stiffness, nausea, vomiting, and photophobia.
In individual cases, especially if dietary guidelines are not observed and there is a drug interaction with other medications, they can cause bleeding in the cranial cavity (intracranial hemorrhage) (see section 2. "Taking tranilcipromina with food, drinks, and alcohol" or "Other medications and tranilcipromina").
Rare:may affect up to 1 in 1,000 people:
- anemia (low red blood cell count), reduction in the number of different cells in the blood;
- psychological dependence;
- cerebral convulsive crises;
- swelling due to tissue fluid accumulation (edema);
- constipation, diarrhea;
- sweating;
- muscle spasms, myalgias;
- abnormal orgasms, erectile dysfunction, ejaculation disorders;
- hallucinations, confusion (rare/very rare);
- neuralgias (polyneuropathies) (rare/very rare);
- blurred vision (rare/very rare);
- hepatic insufficiency, increased hepatic enzyme activity (rare/very rare);
- allergic skin rashes (rare/very rare);
- joint pain (rare/very rare);
- increased body temperature (rare/very rare).
Very rare:may affect up to 1 in 10,000 people:
- alopecia;
- increased release of the ADH hormone, which regulates water balance, with consequent reduction in urination.
Frequency not known:the frequency cannot be estimated from the available data
- suicidal thoughts, suicidal behavior*
- Suicidal thoughts and behaviors have been reported during therapy with medications containing the same active ingredient as Tranilcipromina or shortly after ending treatment (see section 2. "Warnings and precautions").
- tremors, somnolence, and dizziness;
- tinnitus (ringing in the ears);
- nausea with or without vomiting and nonspecific gastrointestinal symptoms;
- muscle contractions;
- urinary disorders;
- chest pain, feeling of cold, and fatigue.
Reporting adverse effects
If you experience any type of adverse effect, consult your doctor or pharmacist, even if it is a possible adverse effect that does not appear in this prospectus. You can also report them directly through the Spanish Pharmacovigilance System for Human Use Medications: https://www.notificaram.es. By reporting adverse effects, you can contribute to providing more information on the safety of this medication.
5. Conservation of Tranilcipromina Aristo
Keep this medication out of sight and reach of children.
Do not use this medication after the expiration date that appears on the box and blister pack after "exp.". The expiration date is the last day of the month indicated.
Do not store at a temperature above 25°C.
Medications should not be thrown away through the sewers or in the trash. Deposit the packaging and medications you no longer need in the SIGRE Point of the pharmacy. In case of doubt, ask your pharmacist how to dispose of the packaging and medications you no longer need. This way, you will help protect the environment.
6. Container Content and Additional Information
Composition of Tranilcipromina
- The active ingredient is tranilcipromina.
Each film-coated tablet contains 20 mg of tranilcipromina (as tranilcipromina sulfate).
- The other components are:
Core of the tablet: lactose monohydrate, corn starch, microcrystalline cellulose, anhydrous colloidal silica.
Coating: poly(vinyl alcohol), Macrogol 3350, titanium dioxide, talc,
yellow iron oxide (E 172), black iron oxide (E 172), carmine (E 132).
Appearance of the Product and Container Content
Tranilcipromina Aristo 20 mg are film-coated tablets, green, round, with a pressure break line and a score.
The tablet can be divided into equal doses.
Blister pack (PVC/PE/ACLAR with child-resistant aluminum foil or PVC/PVDC film with child-resistant aluminum foil).
Tranilcipromina Aristo 20 mg is available in packs of 30, 45, 50, 60, 90 and 100 film-coated tablets.
Not all pack sizes may be marketed.
Marketing Authorization Holder and Manufacturer
Aristo Pharma GmbH
Wallenroder Straße 8-10
13435 Berlin, Germany
You can request more information about this medicinal product from the local representative of the marketing authorization holder:
Aristo Pharma Iberia, S.L.
C/ Solana, 26
28850, Torrejón de Ardoz
Madrid, Spain
This medicinal product is authorized in the Member States of the European Economic Area under the following names:
Austria Tranylcypromin Aristo 20 mg Filmtabletten
Netherlands Tranylcypromine Aristo 20 mg filmomhulde tabletten
Spain Tranilcipromina Aristo 20 mg film-coated tablets
Date of the last revision of this leaflet:10/2022
Detailed information on this medicinal product is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
Annex
Please note the following indication: take only one food per meal, which is allowed in small quantities.
The list is not exhaustive and regional specificities may apply.
Prohibited | Permitted in small quantities | Permitted | |
Milk and dairy products |
|
|
|
Meat and meat products |
|
|
|
Fish and fish products |
|
|
|
Eggs and egg products |
|
| |
Yeast and yeast products |
|
|
|
Cereals and cereal products |
|
| |
Legumes |
|
| |
Cocoa and cocoa products |
of cognac, liqueur-filled chocolates, cocoa liqueur
|
|
|
Fruit |
|
|
|
Vegetables and vegetable products |
|
|
|
Nuts and nut products |
|
|
|
Drinks |
|
|
|
Other food products |
|
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to TRANILCIPROMINE ARISTO 20 mg FILM-COATED TABLETSDosage form: TABLET, 10 mgActive substance: tranylcypromineManufacturer: Aristo Pharma GmbhPrescription requiredDosage form: TABLET, 15 mgActive substance: mirtazapineManufacturer: Laboratorios Alter S.A.Prescription requiredDosage form: TABLET, 30 mgActive substance: mirtazapineManufacturer: Laboratorios Alter S.A.Prescription required
Online doctors for TRANILCIPROMINE ARISTO 20 mg FILM-COATED TABLETS
Discuss questions about TRANILCIPROMINE ARISTO 20 mg FILM-COATED TABLETS, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions











