
TAPENTADOL RETARD STADA 150 mg PROLONGED-RELEASE TABLETS

How to use TAPENTADOL RETARD STADA 150 mg PROLONGED-RELEASE TABLETS
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Tapentadol retardStada25 mg prolonged-release tablets EFG
Tapentadol retard Stada 50 mg prolonged-release tablets EFG
Tapentadol retard Stada 100 mg prolonged-release tablets EFG
Tapentadol retard Stada 150 mg prolonged-release tablets EFG
Tapentadol retard Stada 200 mg prolonged-release tablets EFG
Tapentadol retard Stada 250 mg prolonged-release tablets EFG
Read the entire package leaflet carefully before you start taking this medicine, because it contains important information for you.
- Keep this package leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this package leaflet. See section 4.
Contents of the package leaflet
- What is Tapentadol retard Stada and what is it used for
- What you need to know before you take Tapentadol retard Stada
- How to take Tapentadol retard Stada
- Possible side effects
- Storage of Tapentadol retard Stada
- Package contents and further information
1. What is Tapentadol retard Stada and what is it used for
Tapentadol - the active substance of Tapentadol retard Stada - is a potent analgesic belonging to the class of opioids. Tapentadol is used for the treatment of severe chronic pain in adults, which can only be adequately managed with an opioid analgesic.
2. What you need to know before you take Tapentadol retard Stada
Do not take Tapentadol retard Stada
- if you are allergic to tapentadol or any of the other ingredients of this medicine (listed in section 6)
- if you have asthma or if your breathing is slow or shallow to dangerous levels (respiratory depression, hypercapnia)
- if you have paralytic ileus
- if you are suffering from acute intoxication with alcohol, sleeping pills, analgesics, or other psychotropic medicines (medicines that affect mood and emotions) (see “Other medicines and Tapentadol retard Stada”)
Warnings and precautions
Consult your doctor or pharmacist before starting Tapentadol retard Stada:
- if your breathing is slow or shallow,
- if you have increased intracranial pressure or altered consciousness up to coma,
- if you have had a head injury or brain tumors,
- if you have liver or kidney disease (see “How to take Tapentadol retard Stada”),
- if you have pancreas or bile duct disease, including pancreatitis,
- if you are taking medicines called mixed opioid agonist/antagonists (e.g., pentazocine, nalbuphine) or partial agonists of opioid mu receptors (e.g., buprenorphine),
- if you are prone to epilepsy or seizures or if you are taking other medicines with a known risk of increasing seizures, as the risk of these crises may increase,
- if you or a family member have a history of abuse or dependence on alcohol, prescription drugs, or illicit substances ("addiction"),
- if you smoke,
- if you have ever had problems with your mood (depression, anxiety, or personality disorder) or have received psychiatric treatment for other mental illnesses.
This medicine contains tapentadol, which is an opioid medicine. Repeated use of opioid analgesics may lead to a decrease in their effectiveness (you may get used to it). It can also lead to dependence and abuse, which can result in a potentially life-threatening overdose. It is essential that you inform your doctor if you think you may have developed dependence on tapentadol. Its use (even at therapeutic doses) can cause physical dependence, which may cause you to experience withdrawal symptoms and a recurrence of your problems if you stop taking this treatment suddenly.
Tapentadol can cause physical and psychological addiction. If you tend to abuse medicines or have drug dependence, you should only take these tablets for short periods and under strict medical supervision.
Respiratory disorders related to sleep
Tapentadol can cause sleep-related respiratory disorders such as sleep apnea (pauses in breathing during sleep) and sleep-related hypoxemia (low oxygen levels in the blood). Symptoms may include pauses in breathing during sleep, nighttime awakenings due to difficulty breathing, difficulty maintaining sleep, or excessive daytime sleepiness. If you or someone else observes these symptoms, consult your doctor. Your doctor may consider reducing the dose.
Other medicines and Tapentadol retard Stada
Tell your doctor or pharmacist if you are taking, have recently taken, or might take any other medicines.
- The risk of side effects increases if you are taking medicines that can cause seizures (attacks), such as certain antidepressants or antipsychotics. The risk of seizure crises increases if you take tapentadol simultaneously with these medicines. Your doctor will tell you if tapentadol retard is suitable for you.
- The concomitant use of tapentadol and sedative medicines such as benzodiazepines or related medicines (certain sleeping pills or tranquilizers, e.g., barbiturates, or analgesics like opioids, morphine, and codeine (also as a cough medicine), antipsychotics, antihistamines H1, alcohol) increases the risk of drowsiness, respiratory difficulties (respiratory depression), coma, and can be potentially life-threatening. Due to this, concomitant use should only be considered when other treatment options are not possible. However, if your doctor prescribes tapentadol retard along with sedative medicines, they will limit the dose and duration of concomitant treatment. The concomitant use of opioids and drugs used to treat epilepsy, nerve pain, or anxiety (gabapentin and pregabalin) increases the risk of opioid overdose, respiratory depression, and can be potentially life-threatening. Inform your doctor if you are taking gabapentin or pregabalin or any other sedative medicine and strictly follow your doctor's dosage recommendation. It may be helpful to inform your friends and family about the signs and symptoms indicated above. Contact your doctor if you experience any of these symptoms.
- If you are taking a type of medicine that affects serotonin levels (e.g., certain medicines for treating depression), talk to your doctor before taking tapentadol retard, as there have been cases of "serotonin syndrome". Serotonin syndrome is a rare but potentially life-threatening disorder. Signs may include involuntary muscle contractions, including muscles that control eye movement, agitation, excessive sweating, tremors, exaggerated reflexes, increased muscle tension, and body temperature above 38°C. Your doctor may advise you.
- The concomitant administration of tapentadol with other types of medicines called mixed opioid agonist/antagonists (e.g., pentazocine, nalbuphine) or partial agonists of opioid mu receptors (e.g., buprenorphine) has not been studied. It is possible that tapentadol may not work as well if administered with any of these medicines. Inform your doctor if you are currently being treated with any of these medicines.
- The administration of tapentadol with potent inhibitors or inducers (e.g., rifampicin, phenobarbital, or St. John's Wort) of certain enzymes necessary to eliminate tapentadol from your body may influence the effectiveness of tapentadol or may cause side effects, especially when starting or stopping this other type of medication. Please keep your doctor informed about all the medicines you are taking.
- Tapentadol should not be taken with MAO inhibitors (certain medicines for treating depression). Inform your doctor if you are taking MAO inhibitors or if you have taken them in the last 14 days.
Taking Tapentadol retard Stada with food, drinks, and alcohol
Do not consume alcohol while taking tapentadol, as some side effects such as drowsiness may increase. The intake of food does not affect the effect of this medicine.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medicine.
Do not take this medicine:
- if you are pregnant, unless your doctor has told you to, as tapentadol may cause withdrawal symptoms in the newborn, which can be life-threatening if not detected and treated by a doctor.
- during breastfeeding, as it may be excreted in breast milk.
Tapentadol is not recommended:
- during labor, as it may cause slow or shallow breathing to dangerous levels (respiratory depression) in the newborn.
Driving and using machines
Ask your doctor if you can drive or use machines during treatment with Tapentadol retard. It is essential that before driving or using machines, you observe how this medicine affects you. Do not drive or use machines if you feel drowsy, dizzy, have blurred vision, or double vision, or have difficulty concentrating. Be particularly careful at the start of treatment, after a dose change, and when taking it with alcohol or tranquilizers.
3. How to take Tapentadol retard Stada
Follow exactly the administration instructions of this medicine given by your doctor or pharmacist. In case of doubt, consult your doctor or pharmacist again.
Your doctor will adjust the dose according to the intensity of your pain and your personal sensitivity to pain. Generally, you should take the lowest dose to relieve the pain.
Adults
The recommended initial dose is 50 mg twice a day, approximately every 12 hours.
Daily doses above 500 mg of tapentadol are not recommended.
Your doctor may prescribe a different dose or dosing regimen, more suitable for you if necessary. If you think the effect of these tablets is too strong or too weak, talk to your doctor or pharmacist.
Elderly patients
In elderly patients (over 65 years of age), the dose does not usually need to be adjusted. However, the elimination of tapentadol may be delayed in some patients in this age group. If this applies to you, your doctor may prescribe a different dosing regimen.
Liver and kidney diseases (insufficiency)
Patients with severe liver problems should not take these tablets. If you have moderate liver problems, your doctor will prescribe a different dosing regimen. In case of mild liver problems, no dose adjustment is necessary.
Patients with severe kidney problems should not take these tablets. In case of mild or moderate kidney problems, no dose adjustment is necessary.
Use in children and adolescents
Tapentadol retard is not indicated in children and adolescents under 18 years of age.
How and when to take Tapentadol retard Stada
Tapentadol retard should be taken orally.
Always swallow the tablet with a sufficient amount of liquid. Do not chew or crush it, as this could lead to an overdose because the active substance will be released in your body too quickly. You can take the tablets with or without food.
The tablet can be divided into equal doses.
It is possible that the tablet coating may not be completely digested and, therefore, be visible in the feces. This should not worry you, as the medicine (active substance) of the tablet will have already been absorbed by the body, and what you see is only the empty coating.
Instructions for opening the blister
This medicine is packaged in child-resistant, single-dose precut blisters. You cannot push the tablet through the blister. Please follow the instructions for opening the blister:
- Cut a dose along the precut lines of the blister.
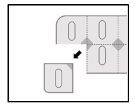
- Locate the non-sealed area where the precut lines cross.

- Pull the non-sealed area to peel off the foil

For how long you should take Tapentadol retard Stada
Do not take the tablets for longer than your doctor has told you.
If you take more Tapentadol retard Stada than you should
After taking very high doses, you may experience some of the following effects:
- pupils very reduced
- vomiting
- decreased blood pressure
- rapid heartbeat
- fainting, altered consciousness, or coma (deep loss of consciousness)
- seizures
- slow or shallow breathing to dangerous levels or respiratory arrest.
If you experience any of these effects, you should contact a doctor immediately.
In case of overdose or accidental ingestion, consult your doctor or pharmacist immediately or call the Toxicology Information Service, phone: 91 562 04 20, indicating the medicine and the amount used. It is recommended to take the package and the package leaflet of the medicine to the healthcare professional.
If you forget to take Tapentadol retard Stada
If you forget to take a tablet, you will probably feel pain again. Do not take a double dose to make up for forgotten doses; simply continue taking the tablets as before.
If you stop taking Tapentadol retard Stada
If you stop or discontinue treatment too early, you will probably feel pain again. If you want to stop treatment, consult your doctor before doing so.
Generally, no withdrawal symptoms occur after stopping treatment; however, in rare cases, people who have taken the tablets for a long time may feel unwell if they stop taking them suddenly.
Symptoms may be:
- restlessness, tearful eyes, runny nose, yawning, sweating, chills, muscle pain, and dilated pupils,
- irritability, anxiety, back pain, joint pain, weakness, abdominal cramps,
difficulty sleeping, nausea, loss of appetite, vomiting, diarrhea, and increases in blood pressure, respiratory rate, or heart rate.
If you experience any of these symptoms after stopping treatment, please consult your doctor.
You should not stop this medicine abruptly, unless your doctor tells you to. If your doctor wants you to stop taking these tablets, they will tell you how to do it, which may involve gradually reducing the dose.
If you have any further questions about the use of this medicine, ask your doctor or pharmacist.
4. Possible Adverse Effects
Like all medicines, this medicine can cause adverse effects, although not all people suffer from them.
Adverse effects or important symptoms to be aware of and what to do if you are affected by them:
- This medicine may cause allergic reactions. Symptoms may include wheezing, difficulty breathing, swelling of the eyelids, face or lips, skin rash or itching, especially if they affect the whole body.
- Another serious adverse effect is breathing more slowly or more weakly than normal. It occurs mostly in elderly patients or weakened patients.
If you experience any of these important symptoms, consult your doctor immediately.
Other adverse effects that may occur:
Very common (may affect more than 1 in 10 people)
- nausea, constipation
- dizziness, drowsiness, headache
Common (may affect up to 1 in 10 people)
- decreased appetite, anxiety, depression, difficulty sleeping, nervousness, restlessness, attention disorders,
- tremors, muscle twitches,
- hot flashes,
- shortness of breath,
- vomiting, diarrhea, indigestion,
- itching, increased sweating, skin rashes,
- feeling of weakness, fatigue, feeling of change in body temperature, dryness of mucous membranes, water accumulation in tissues (edema).
Uncommon (may affect up to 1 in 100 people)
- drug allergy (including swelling under the skin, urticarial habon and in severe cases difficulty breathing, decreased blood pressure, collapse or shock),
- weight loss,
- disorientation, confusion, excitability (agitation), perception disorders, sleep disorders, euphoric mood, decreased level of consciousness, memory impairment, mental deterioration,
- fainting, sedation, balance disorders, speech difficulties, numbness, abnormal sensations in the skin (e.g., tingling, itching),
- vision disorders,
- rapid heartbeats, slow heartbeats, palpitations, decreased blood pressure,
- abdominal discomfort,
- rash,
- delayed urination, frequent urination,
- sexual dysfunction,
- drug withdrawal syndrome (see "If you stop treatment with Tapentadol retard Stada"), feeling of discomfort, irritability.
Rare (may affect up to 1 in 1,000 people)
- drug dependence, thought disorder, epileptic seizures, feeling of being about to faint, altered coordination,
- slow or shallow breathing to dangerous levels (respiratory depression),
- gastric emptying disorder,
- feeling of intoxication, feeling of relaxation.
Frequency not known (cannot be estimated from available data)
- delirium
In general, the possibility of having suicidal thoughts and behaviors increases in patients with chronic pain. Additionally, some medications for treating depression (with an impact on the brain's neurotransmitter system) may increase this risk, especially at the start of treatment. Although tapentadol also affects neurotransmitters, experience in patients has not proven that it increases this risk.
Reporting of Adverse Effects
If you experience any type of adverse effect, consult your doctor or pharmacist, even if it is a possible adverse effect that does not appear in this prospectus. You can also report them directly through the Spanish Pharmacovigilance System for Human Use Medicines: www.notificaram.es. By reporting adverse effects, you can contribute to providing more information on the safety of this medicine.
5. Storage of Tapentadol retard Stada
Keep this medicine out of sight and reach of children.
Do not use this medicine after the expiration date that appears on the packaging and the blister after CAD. The expiration date is the last day of the month indicated.
This medicine does not require special storage conditions.
Medicines should not be thrown away through drains or into the trash. Deposit the packaging and medicines you no longer need at the SIGRE Point in the pharmacy. Ask your pharmacist how to dispose of the packaging and medicines you no longer need. This way, you will help protect the environment.
6. Package Contents and Additional Information
Composition of Tapentadol retard Stada
The active ingredient is tapentadol.
Tapentadol retard Stada 25 mg prolonged-release tablets EFG
Each prolonged-release tablet contains tapentadol phosphate equivalent to 25 mg of tapentadol.
Tapentadol retard Stada 50 mg prolonged-release tablets EFG
Each prolonged-release tablet contains tapentadol phosphate equivalent to 50 mg of tapentadol.
Tapentadol retard Stada 100 mg prolonged-release tablets EFG
Each prolonged-release tablet contains tapentadol phosphate equivalent to 100 mg of tapentadol.
Tapentadol retard Stada 150 mg prolonged-release tablets EFG
Each prolonged-release tablet contains tapentadol phosphate equivalent to 150 mg of tapentadol.
Tapentadol retard Stada 200 mg prolonged-release tablets EFG
Each prolonged-release tablet contains tapentadol phosphate equivalent to 200 mg of tapentadol.
Tapentadol retard Stada 250 mg prolonged-release tablets EFG
Each prolonged-release tablet contains tapentadol phosphate equivalent to 250 mg of tapentadol.
The other components (excipients) are:
Core of the tablet: microcrystalline cellulose (E460), hypromellose (E464), anhydrous colloidal silica (E551), magnesium stearate.
Coating of the tablet: hypromellose (E464), glycerol (E422), talc (E553b), microcrystalline cellulose (E460), titanium dioxide (E171), red iron oxide (only in 25, 100, 150, 200, and 250 mg doses) (E172), yellow iron oxide (only in 25, 100, and 200 mg doses) (E172), black iron oxide (only in 25, 100, 150, 200, and 250 mg doses) (E172).
Appearance of the Product and Package Contents
Tapentadol retard Stada 25 mg prolonged-release tablets EFG are brownish, oblong, biconvex (6 mm x 12 mm) tablets with a score line on both sides.
The tablet can be divided into equal doses.
Tapentadol retard Stada 50 mg prolonged-release tablets EFG are white, oblong, biconvex (6 mm x 13 mm) tablets with a score line on both sides.
The tablet can be divided into equal doses.
Tapentadol retard Stada 100 mg prolonged-release tablets EFG are yellowish, oblong, biconvex (7 mm x 14 mm) tablets with a score line on both sides.
The tablet can be divided into equal doses.
Tapentadol retard Stada 150 mg prolonged-release tablets EFG are bright reddish, oblong, biconvex (7 mm x 15 mm) tablets with a score line on both sides.
The tablet can be divided into equal doses.
Tapentadol retard Stada 200 mg prolonged-release tablets EFG are yellow, oblong, biconvex (8 mm x 16 mm) tablets with a score line on both sides.
The tablet can be divided into equal doses.
Tapentadol retard Stada 250 mg prolonged-release tablets EFG are reddish-brown, oblong, biconvex (9 mm x 18 mm) tablets with a score line on both sides.
The tablet can be divided into equal doses.
Tapentadol retard Stada is available in the following formats:
Tapentadol retard Stada 25 mg prolonged-release tablets EFG
20x1, 30x1, 40x1, 50x1, 54x1, 60x1, or 100x1 tablets in child-resistant single-dose blister packs.
Tapentadol retard Stada 50, 100, 150, 200, 250 mg prolonged-release tablets EFG
20x1, 24x1, 30x1, 50x1, 54x1, 60x1, or 100x1 tablets in child-resistant single-dose blister packs.
Not all pack sizes may be marketed.
Marketing Authorization Holder and Manufacturer
Marketing Authorization Holder
Laboratorio STADA, S.L.
Frederic Mompou, 5
08960 Sant Just Desvern (Barcelona)
Spain
Manufacturer
Develco Pharma GmbH
Grienmatt 27
79650 Schopfheim
Germany
or
STADA Arzneimittel AG
Stadastrasse 2 – 18
61118 Bad Vilbel
Germany
or
Centrafarm Services B.V.,
Van de Reijtstraat 31-E,
4814 NE Breda,
Netherlands
This medicine is authorized in the Member States of the European Economic Area under the following names:
Germany | Tapentadol AL 25 mg Retardtabletten Tapentadol AL 50 mg Retardtabletten Tapentadol AL 100 mg Retardtabletten Tapentadol AL 150 mg Retardtabletten Tapentadol AL 200 mg Retardtabletten Tapentadol AL 250 mg Retardtabletten |
Croatia | TAPISTA |
Denmark | Tapentadol STADA |
Slovakia | Tapestad retard 50 mg, 100 mg, 150 mg, 200 mg, 250 mg |
Spain | Tapentadol retard STADA 25 mg prolonged-release tablets EFG Tapentadol retard STADA 50 mg prolonged-release tablets EFG Tapentadol retard STADA 100 mg prolonged-release tablets EFG Tapentadol retard STADA 150 mg prolonged-release tablets EFG Tapentadol retard STADA 200 mg prolonged-release tablets EFG Tapentadol retard STADA 250 mg prolonged-release tablets EFG |
Iceland | Tapentadol STADA 25 mg, 50 mg, 100 mg, 150 mg, 200 mg, 250 mg; forðahylki |
Italy | Mudol |
Norway | Tapentadol STADA |
Netherlands | Tapentadol retard CF 25 mg, tabletten met verlengde afgifte Tapentadol retard CF 50 mg, tabletten met verlengde afgifte Tapentadol retard CF 100 mg, tabletten met verlengde afgifte Tapentadol retard CF 150 mg, tabletten met verlengde afgifte Tapentadol retard CF 200 mg, tabletten met verlengde afgifte Tapentadol retard CF 250 mg, tabletten met verlengde afgifte |
Poland | BINATTA |
Czech Republic | Taxemba |
Sweden | Tapentadol Depot STADA 25 mg, 50 mg, 100 mg, 150 mg, 200 mg, 250 mg; depottabletter |
Date of the last revision of this prospectus:September 2022.
Detailed and updated information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) (http://www.aemps.gob.es/).
- Country of registration
- Average pharmacy price66.03 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to TAPENTADOL RETARD STADA 150 mg PROLONGED-RELEASE TABLETSDosage form: MODIFIED-RELEASE TABLET, 100 mgActive substance: tapentadolManufacturer: Sandoz Farmaceutica S.A.Prescription requiredDosage form: MODIFIED-RELEASE TABLET, 150 mgActive substance: tapentadolManufacturer: Sandoz Farmaceutica S.A.Prescription requiredDosage form: MODIFIED-RELEASE TABLET, 200 mgActive substance: tapentadolManufacturer: Sandoz Farmaceutica S.A.Prescription required
Online doctors for TAPENTADOL RETARD STADA 150 mg PROLONGED-RELEASE TABLETS
Discuss questions about TAPENTADOL RETARD STADA 150 mg PROLONGED-RELEASE TABLETS, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















