TALVEY 2 mg/ml INJECTABLE SOLUTION

How to use TALVEY 2 mg/ml INJECTABLE SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Talvey 2 mg/ml injectable solution
Talvey 40 mg/ml injectable solution
Talquetamab
This medicine is subject to additional monitoring, which will allow for quick identification of new safety information. You can help by reporting any side effects you may get. The last section of this leaflet includes information on how to report side effects.
Read all of this leaflet carefully before you start receiving this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or nurse.
- If you get any side effects, talk to your doctor or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Talvey and what is it used for
- What you need to know before you receive Talvey
- How to receive Talvey
- Possible side effects
- Storage of Talvey
- Contents of the pack and further information
1. What is Talvey and what is it used for
Talvey is a cancer medicine that contains the active substance talquetamab. Talquetamab is an antibody, a type of protein that recognizes specific targets in your body and binds to them. It has been designed to bind to the protein GPRC5D (G-protein coupled receptor, family C, group 5, and member D), which is found on multiple myeloma cancer cells, and to the cluster of differentiation 3 (CD3), a protein on so-called "T-lymphocytes" (a type of white blood cell). T-lymphocytes are part of the body's natural defenses and help protect it from infections.
They can also destroy cancer cells. When this medicine binds to these cells, it brings cancer cells and T-cells together. This stimulates T-cells to destroy multiple myeloma cancer cells.
Talvey is used to treat adults with multiple myeloma, a cancer of the bone marrow.
It is used when patients have had at least three other types of treatment that either have not worked or have stopped working.
2. What you need to know before you receive Talvey
Do not receive Talvey
- if you are allergic to talquetamab or any of the other ingredients of this medicine (listed in section 6).
Do not use Talvey if any of the above applies to you. If you are not sure, talk to your doctor or nurse before receiving Talvey.
Warnings and precautions
Talk to your doctor or nurse before starting to receive Talvey.
Severe side effects
There are severe side effects that can occur after starting to take Talvey. You should immediately inform your doctor or nurse if this happens, as you may need immediate medical attention.
Immediately inform your doctor or nurse if you experience any of the following:
- signs of a condition known as "cytokine release syndrome" (CRS). CRS is a severe immune reaction in which symptoms such as fever, low blood pressure, chills, difficulty breathing, fatigue, headache, rapid heart rate, and increased liver enzyme levels in the blood occur.
- effects on the nervous system. Symptoms include feeling confused, disoriented, drowsy, lacking attention, slow or having difficulty thinking, altered thinking or decreased consciousness, confusion, difficulty speaking and understanding speech. Some of these may be signs of a severe immune reaction called "immune effector cell-associated neurotoxicity syndrome" (ICANS).
- problems in the mouth, such as loss of taste, dry mouth, difficulty swallowing, and inflammation of the lining of the mouth.
- skin problems such as rash, redness, and nail problems.
- a feeling of warmth, fever, chills, sore throat, or ulcers in the mouth may be signs of an infection.
Talvey and vaccines
Talk to your doctor or nurse before you are given Talvey if you have recently been vaccinated or are going to be vaccinated. Your immune system (the body's natural defenses) may not respond as well to vaccination when you are taking this medicine.
You should not receive live vaccines, a specific type of vaccine, from at least 4 weeks before starting your treatment with Talvey until at least 4 weeks after you have taken your last dose.
Tests and checks
Beforeyou are given Talvey, your doctor will do a blood test to check the levels of different blood cells and detect signs of infection. Infections will be treated before you start receiving this medicine.
Afterreceiving Talvey, your doctor will check you for side effects. They will also regularly check your blood counts, as the number of blood cells and other components of the blood may decrease when using this medicine.
Children and adolescents
Talvey should not be used in children or adolescents under 18 years of age, as the medicine has not been studied in this age group and it is not known how this medicine may affect them.
Other medicines and Talvey
Tell your doctor or nurse if you are taking, have recently taken, or might take any other medicines. This includes medicines that you can buy without a prescription and herbal products.
Pregnancy, contraception, and breastfeeding
Pregnancy and contraception
Talvey has the potential to be transmitted from the mother to the developing fetus. The effects of Talvey on the developing fetus are unknown and cannot be excluded as a risk for newborns/infants.
If you are pregnant, think you may be pregnant, or plan to become pregnant, consult your doctor or nurse before using this medicine.
If you become pregnant while being treated with this medicine, inform your doctor or nurse immediately.
If you can become pregnant, you must use an effective method of contraception during treatment and for 3 months after stopping treatment with Talvey. Your doctor will check if you are pregnant before starting treatment.
If your partner becomes pregnant while you are taking this medicine, inform your doctor immediately.
If you have received this medicine during pregnancy, your newborn baby should not receive any live vaccine until they are at least 4 weeks old.
Breastfeeding
It is not known if Talvey can pass into breast milk. There may be a risk to newborns or breastfed infants. Consult your doctor before you start receiving this medicine. You and your doctor will decide whether the benefit of breastfeeding is greater than the risk to your baby. If you and your doctor decide to stop receiving this medicine, you should not breastfeed for 3 months after stopping treatment.
Fertility
There are no data on the effect of talquetamab on fertility. The effects of talquetamab on male and female fertility have not been evaluated in animal studies.
Driving and using machines
Some people may feel tired, dizzy, or confused when using Talvey. Do not drive, use tools, or operate machinery from the time you receive your first dose until at least 48 hours after receiving your first dose of Talvey, or as directed by your doctor.
Talvey contains sodium
Talvey contains less than 1 mmol of sodium (23 mg) per dose; this is essentially "sodium-free".
3. How to receive Talvey
Dose administered
Talvey will be administered to you under the supervision of a doctor with experience in treating patients with multiple myeloma. Your doctor will determine how much Talvey you will receive. The dose of Talvey will depend on your body weight.
Talvey is administered once a week or once every 2 weeks, depending on the dose, as follows:
0.4 mg/kg once a week:
- In the first dose, you will receive 0.01 mg per kilogram of body weight.
- In the second dose, which will be administered 2-4 days later, you will receive 0.06 mg per kilogram of body weight.
- In the third dose, you will receive a "treatment dose" of 0.4 mg per kilogram of body weight 2-4 days after the second dose.
- After the third dose, you will then receive a "treatment dose" once a week from then on.
- Treatment will continue as long as you benefit from using Talvey.
Your doctor will check you for side effects after each of your first three doses.
They will do this for 2 days after each dose. You should stay near a medical center after each of your first three doses in case you have side effects.
If you experience side effects after either of your first two doses, your doctor may decide to wait until 7 days before giving you the next dose.
0.8 mg/kg once every 2 weeks:
- In the first dose, you will receive 0.01 mg per kilogram of body weight.
- In the second dose, which will be administered 2-4 days later, you will receive 0.06 mg per kilogram of body weight.
- In the third dose, which will be administered 2-4 days later, you will receive 0.4 mg per kilogram of body weight.
- In the fourth dose, you will then receive a "treatment dose" of 0.8 mg per kilogram of body weight 2-4 days after the third dose.
- After the fourth dose, you will then receive a "treatment dose" once every 2 weeks from then on.
- Treatment will continue as long as you benefit from using Talvey.
Your doctor will check you for side effects after each of your first four doses. They will do this for 2 days after each dose. You should stay near a medical center after each of your first four doses in case you have side effects.
If you experience side effects after any of your first three doses, your doctor may decide to wait until 7 days before giving you the next dose.
The decision to use 0.4 mg/kg once a week or 0.8 mg/kg every 2 weeks should be made in consultation with your doctor.
How the medicine is administered
Talvey will be administered to you by a doctor or nurse as an injection under your skin (subcutaneous injection). It is administered in the stomach area (abdomen) or in the thigh.
Other medicines given during treatment with Talvey
Before the first three doses (if you are receiving 0.4 mg/kg of body weight) or the first four doses (if you are receiving 0.8 mg/kg of body weight) of Talvey, you will be given medicines that will help reduce the possibility of side effects. These may include:
- medicines to reduce an allergic reaction (antihistamines)
- medicines to reduce inflammation (corticosteroids)
- medicines to reduce fever (such as paracetamol)
You may also be given these medicines when you receive subsequent doses of Talvey depending on the symptoms you have.
You may also be given additional medicines depending on the symptoms you have or your medical history.
If you are given more Talvey than you should
This medicine will be administered to you by your doctor or nurse. In case you are given too much (an overdose), your doctor will check you for side effects.
If you miss your appointment for the administration of Talvey
It is very important to attend all appointments for the treatment to work. If you miss an appointment, make another as soon as possible.
If you have any other questions about the use of this medicine, ask your doctor or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Severe side effects
Seek medical attention immediately if you experience any of the following severe side effects, which can be serious and even life-threatening.
Very common (may affect more than 1 in 10 people):
- Immune effector cell-associated neurotoxicity syndrome (ICANS), a severe immune reaction that can affect your nervous system. Some of the symptoms are:
- feeling confused
- feeling less alert or conscious
- disorientation
- drowsiness
- lack of energy
- slowness and difficulty thinking.
- Cytokine release syndrome (CRS), a severe immune reaction. CRS can cause symptoms such as
- fever
- low blood pressure
- chills
- low oxygen level in the blood
- headache
- rapid heart rate
- increased liver enzyme levels in the blood
- low levels of neutrophils (neutropenia), a type of white blood cell that helps fight infections
- reduced number of "platelets" in the blood (thrombocytopenia), which help blood clot;
Tell your doctor immediately if you experience any of the above severe side effects.
Other side effects
The following are other side effects. If you experience any of these side effects, tell your doctor or nurse.
Very common (may affect more than 1 in 10 people):
- nail problems
- muscle and bone pain (musculoskeletal pain)
- low red blood cell count (anemia)
- feeling tired
- chills
- weight loss
- dry skin or mucous membranes, such as the mouth and eyes (xerosis)
- low levels of lymphocytes (lymphopenia), a type of white blood cell
- problems with movement (motor dysfunction)
- feeling dizzy
- nerve damage that can cause tingling, numbness, pain, or loss of pain sensation (sensory neuropathy)
- brain damage or disease that affects brain function (encephalopathy)
- diarrhea
- nausea
- constipation
- stomach pain
- vomiting
- infection of the nose, sinuses, or throat (upper respiratory tract infection)
- itching (pruritus)
- decreased appetite
- pain
- low white blood cell count (leukopenia)
- low levels of "potassium" in the blood (hypokalemia)
- low levels of "phosphate" in the blood (hypophosphatemia)
- low levels of "magnesium" in the blood (hypomagnesemia)
- low levels of immunoglobulins, a type of antibody in the blood (hypogammaglobulinemia), which can increase the risk of infections
- swelling caused by fluid accumulation in the body (edema)
- irritation or pain at the injection site
- increased liver enzymes in the blood
- COVID-19 infection
- blood tests may show that the blood takes longer to clot (decreased fibrinogen, elevated INR, and prolonged TTPa)
- bacterial infection
- mouth pain
- fungal infection
- fever (pyrexia)
- headache
- difficulty breathing (dyspnea)
- cough
- problems with the mouth and swallowing, such as changes in taste (dysgeusia), dry mouth, difficulty swallowing (dysphagia), and inflammation of the lining of the mouth (stomatitis)
- skin problems, including rash
Common (may affect up to 1 in 10 people)
- hair loss
- bleeding, which can be severe (hemorrhages)
- lung infection (pneumonia)
- viral infection
- blood infection (sepsis)
- low levels of a type of white blood cell (neutrophils) with fever
Reporting of side effects
If you experience any side effects, talk to your doctor or nurse, even if they are not listed in this leaflet. You can also report side effects directly through the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Talvey
Talvey will be stored by your doctor in the hospital or medical center. Therefore, the following information is mainly intended for healthcare professionals.
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the carton and on the label of the vial after "EXP". The expiry date is the last day of the month shown.
Store in a refrigerator (between 2°C and 8°C). Do not freeze.
Store in the original package to protect from light.
Before using this medicine, check that the solution does not contain particles or discoloration. The solution should be colorless to pale yellow. Do not use this medicine if you notice it is cloudy, discolored, or contains visible particles.
Medicines should not be disposed of via wastewater or household waste. Your healthcare professional will dispose of any unused medicines. This will help protect the environment.
6. Container Contents and Additional Information
Talvey Composition
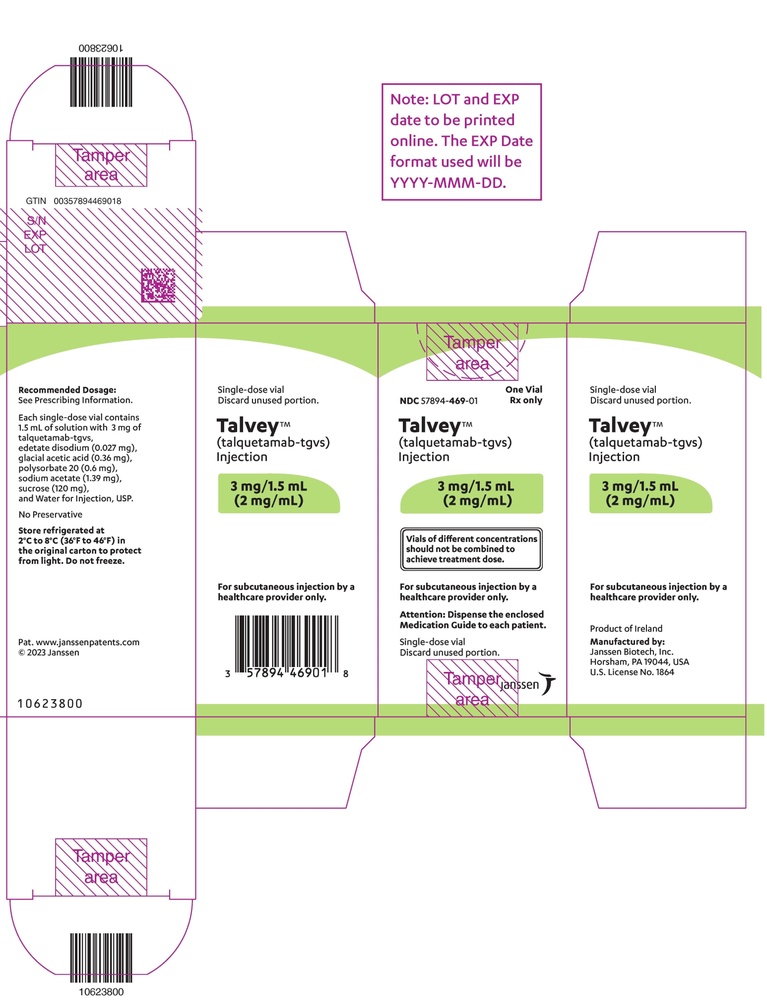
- The active ingredient is talquetamab. Talvey is available in two different concentrations:
- 2 mg/ml – a 1.5 ml vial contains 3 mg of talquetamab
- 40 mg/ml – a 1 ml vial contains 40 mg of talquetamab
- The other components are disodium dihydrate EDTA (E385), glacial acetic acid (E260), polysorbate 20 (E432), sodium acetate trihydrate (E262), sucrose (E473), and water for injectable preparations (see "Talvey contains sodium" in section 2).
Appearance of Talvey and Container Contents
Talvey is a colorless to light yellow liquid injectable solution.
Talvey is presented in a cardboard container containing 1 glass vial.
Marketing Authorization Holder
Janssen-Cilag International NV
Turnhoutseweg 30
B-2340 Beerse
Belgium
Manufacturer
Janssen Biologics B.V.
Einsteinweg 101
2333 CB Leiden
Netherlands
Janssen Pharmaceutica NV
Turnhoutseweg 30
B-2340 Beerse
Belgium
You can request more information about this medication by contacting the local representative of the marketing authorization holder:
België/Belgique/Belgien Janssen-Cilag NV Tel/Tél: +32 14 64 94 11 | Lietuva UAB "JOHNSON & JOHNSON" Tel: +370 5 278 68 88 |
| Luxembourg/Luxemburg Janssen-Cilag NV Tél/Tel: +32 14 64 94 11 |
Ceská republika Janssen-Cilag s.r.o. Tel: +420 227 012 227 | Magyarország Janssen-Cilag Kft. Tel.: +36 1 884 2858 |
Danmark Janssen-Cilag A/S Tlf.: +45 4594 8282 | Malta AM MANGION LTD Tel: +356 2397 6000 |
Deutschland Janssen-Cilag GmbH Tel: 0800 086 9247 / +49 2137 955 6955 | Nederland Janssen-Cilag B.V. Tel: +31 76 711 1111 |
Eesti UAB "JOHNSON & JOHNSON" Eesti filiaal Tel: +372 617 7410 | Norge Janssen-Cilag AS Tlf: +47 24 12 65 00 |
| Österreich Janssen-Cilag Pharma GmbH Tel: +43 1 610 300 |
España Janssen-Cilag, S.A. Tel: +34 91 722 81 00 | Polska Janssen-Cilag Polska Sp. z o.o. Tel.: +48 22 237 60 00 |
France Janssen-Cilag Tél: 0 800 25 50 75 / +33 1 55 00 40 03 | Portugal Janssen-Cilag Farmacêutica, Lda. Tel: +351 214 368 600 |
Hrvatska Johnson & Johnson S.E. d.o.o. Tel: +385 1 6610 700 | România Johnson & Johnson România SRL Tel: +40 21 207 1800 |
Ireland Janssen Sciences Ireland UC Tel: 1 800 709 122 | Slovenija Johnson & Johnson d.o.o. Tel: +386 1 401 18 00 |
Ísland Janssen-Cilag AB c/o Vistor hf. Sími: +354 535 7000 | Slovenská republika Johnson & Johnson, s.r.o. Tel: +421 232 408 400 |
Italia Janssen-Cilag SpA Tel: 800.688.777 / +39 02 2510 1 | Suomi/Finland Janssen-Cilag Oy Puh/Tel: +358 207 531 300 |
| Sverige Janssen-Cilag AB Tel: +46 8 626 50 00 |
Latvija UAB "JOHNSON & JOHNSON" filiale Latvija Tel: +371 678 93561 | United Kingdom (Northern Ireland) Janssen Sciences Ireland UC Tel: +44 1 494 567 444 |
Date of Last Revision of this Leaflet:
This medicinal product has been authorized with a "conditional approval". This type of approval means that more information on this medicinal product is expected.
The European Medicines Agency will review the new information on this medicinal product at least once a year, and this leaflet will be updated as necessary.
Other Sources of Information
Detailed information on this medicinal product is available on the European Medicines Agency website: https://www.ema.europa.eu/
On the European Medicines Agency website, this leaflet can be found in all languages of the European Union/European Economic Area.
< -------------------------------------------------------------------------------------------------------------------- >
This information is intended for healthcare professionals only:
Talvey vials are supplied as a ready-to-use injectable solution that does not require dilution before administration.
Vials of Talvey from different concentrations should not be combined to obtain the treatment dose.
To prepare and administer Talvey, aseptic technique should be used.
Preparation of Talvey
- Consult the following reference tables for the preparation of Talvey.
- Use Table 1 to determine the total dose, injection volume, and number of vials required based on the patient's current body weight for the 0.01 mg/kg dose using the Talvey 2 mg/ml vial.
Table 1: 0.01 mg/kg dose: injection volumes with Talvey 2 mg/ml vial
Dose of 0.01 mg/kg | Body weight (kg) | Total doseª (mg) | Injection volume (ml) | Number of vials (1 vial = 1.5 ml) |
35 to 39 | 0.38 | 0.19 | 1 | |
40 to 45 | 0.42 | 0.21 | 1 | |
46 to 55 | 0.5 | 0.25 | 1 | |
56 to 65 | 0.6 | 0.3 | 1 | |
66 to 75 | 0.7 | 0.35 | 1 | |
76 to 85 | 0.8 | 0.4 | 1 | |
86 to 95 | 0.9 | 0.45 | 1 | |
96 to 105 | 1.0 | 0.5 | 1 | |
106 to 115 | 1.1 | 0.55 | 1 | |
116 to 125 | 1.2 | 0.6 | 1 | |
126 to 135 | 1.3 | 0.65 | 1 | |
136 to 145 | 1.4 | 0.7 | 1 | |
146 to 155 | 1.5 | 0.75 | 1 | |
156 to 160 | 1.6 | 0.8 | 1 |
ª The total dose (mg) is calculated based on the rounded injection volume (ml)
- Use Table 2 to determine the total dose, injection volume, and number of vials required based on the patient's current body weight for the 0.06 mg/kg dose using the Talvey 2 mg/ml vial.
Table 2: 0.06 mg/kg dose: injection volumes with Talvey 2 mg/ml vial
Dose of 0.06 mg/kg | Body weight (kg) | Total doseª (mg) | Injection volume (ml) | Number of vials (1 vial = 1.5 ml) |
35 to 39 | 2.2 | 1.1 | 1 | |
40 to 45 | 2.6 | 1.3 | 1 | |
46 to 55 | 3 | 1.5 | 1 | |
56 to 65 | 3.6 | 1.8 | 2 | |
66 to 75 | 4.2 | 2.1 | 2 | |
76 to 85 | 4.8 | 2.4 | 2 | |
86 to 95 | 5.4 | 2.7 | 2 | |
96 to 105 | 6 | 3 | 2 | |
106 to 115 | 6.6 | 3.3 | 3 | |
116 to 125 | 7.2 | 3.6 | 3 | |
126 to 135 | 7.8 | 3.9 | 3 | |
136 to 145 | 8.4 | 4.2 | 3 | |
146 to 155 | 9 | 4.5 | 3 | |
156 to 160 | 9.6 | 4.8 | 4 |
ª The total dose (mg) is calculated based on the rounded injection volume (ml)
- Use Table 3 to determine the total dose, injection volume, and number of vials required based on the patient's current body weight for the 0.4 mg/kg dose using the Talvey 40 mg/ml vial.
Table 3: 0.4 mg/kg dose: injection volumes with Talvey 40 mg/ml vial
Dose of 0.4 mg/kg | Body weight (kg) | Total doseª (mg) | Injection volume (ml) | Number of vials (1 vial = 1.0 ml) |
35 to 39 | 14.8 | 0.37 | 1 | |
40 to 45 | 16 | 0.4 | 1 | |
46 to 55 | 20 | 0.5 | 1 | |
56 to 65 | 24 | 0.6 | 1 | |
66 to 75 | 28 | 0.7 | 1 | |
76 to 85 | 32 | 0.8 | 1 | |
86 to 95 | 36 | 0.9 | 1 | |
96 to 105 | 40 | 1 | 1 | |
106 to 115 | 44 | 1.1 | 2 | |
116 to 125 | 48 | 1.2 | 2 | |
126 to 135 | 52 | 1.3 | 2 | |
136 to 145 | 56 | 1.4 | 2 | |
146 to 155 | 60 | 1.5 | 2 | |
156 to 160 | 64 | 1.6 | 2 |
ª The total dose (mg) is calculated based on the rounded injection volume (ml)
- Use Table 4 to determine the total dose, injection volume, and number of vials required based on the patient's current body weight for the 0.8 mg/kg dose using the Talvey 40 mg/ml vial.
Table 4: 0.8 mg/kg dose: injection volumes with Talvey 40 mg/ml vial
Dose of 0.8 mg/kg | Body weight (kg) | Total doseª (mg) | Injection volume (ml) | Number of vials (1 vial = 1.0 ml) |
35 to 39 | 29.6 | 0.74 | 1 | |
40 to 45 | 34 | 0.85 | 1 | |
46 to 55 | 40 | 1 | 1 | |
56 to 65 | 48 | 1.2 | 2 | |
66 to 75 | 56 | 1.4 | 2 | |
76 to 85 | 64 | 1.6 | 2 | |
86 to 95 | 72 | 1.8 | 2 | |
96 to 105 | 80 | 2 | 2 | |
106 to 115 | 88 | 2.2 | 3 | |
116 to 125 | 96 | 2.4 | 3 | |
126 to 135 | 104 | 2.6 | 3 | |
136 to 145 | 112 | 2.8 | 3 | |
146 to 155 | 120 | 3 | 3 | |
156 to 160 | 128 | 3.2 | 4 |
ª The total dose (mg) is calculated based on the rounded injection volume (ml)
- Check that the Talvey injectable solution is colorless to light yellow. Do not use it if the solution is discolored, cloudy, or if there are foreign particles.
- Remove the Talvey vial of the corresponding concentration from refrigerated storage (2 °C to 8 °C) and equilibrate it to room temperature (15 °C to 30 °C) for at least 15 minutes. Do not heat the Talvey vial in any other way.
- Once equilibrated, gently mix the vial for approximately 10 seconds. Do not shake.
- Withdraw the required injection volume from Talvey from the (from the) vial(s) into a syringe of suitable size using a transfer needle.
- Each injection volume should not exceed 2.0 ml. Divide doses that require more than 2.0 ml evenly into multiple syringes.
- Talvey is compatible with stainless steel injection needles and polypropylene or polycarbonate syringe material.
- Replace the transfer needle with one of suitable size for injection.
Administration of Talvey
- Talvey should be administered by subcutaneous injection.
- Talvey should be administered by a healthcare professional with adequate medical training and with appropriate medical equipment to handle serious reactions, including SLC.
- Inject the required volume of Talvey into the subcutaneous tissue of the abdomen (preferred injection site). Alternatively, Talvey can be injected into the subcutaneous tissue at other sites (e.g., thigh). If multiple injections are required, the Talvey injection sites should be separated by at least 2 cm.
- Do not inject into tattoos or scars or into areas where the skin is red, bruised, sensitive, hard, or not intact.
- The disposal of unused medicinal products and all materials that have come into contact with them will be carried out in accordance with local regulations.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to TALVEY 2 mg/ml INJECTABLE SOLUTIONDosage form: INJECTABLE, 40 mg/mLActive substance: talquetamabManufacturer: Janssen-Cilag International N.VPrescription requiredDosage form: INJECTABLE INFUSION, 50 mgActive substance: brentuximab vedotinManufacturer: Takeda Pharma A/SPrescription requiredDosage form: INJECTABLE PERFUSION, 100 mgActive substance: belantamab mafodotinManufacturer: Glaxosmithkline Trading Services LimitedPrescription required
Online doctors for TALVEY 2 mg/ml INJECTABLE SOLUTION
Discuss questions about TALVEY 2 mg/ml INJECTABLE SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions