
TABRECTA 200 mg FILM-COATED TABLETS

How to use TABRECTA 200 mg FILM-COATED TABLETS
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Prospective: patient information
Tabrecta 150 mg film-coated tablets
Tabrecta 200 mg film-coated tablets
capmatinib
This medicinal product is subject to additional monitoring, which will allow for the rapid detection of new safety information. You can help by reporting any side effects you may have. The last section of section 4 will provide information on how to report side effects.
Read all of this leaflet carefully before you start taking this medicine, because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Tabrecta and what is it used for
- What you need to know before you take Tabrecta
- How to take Tabrecta
- Possible side effects
- Storage of Tabrecta
- Contents of the pack and further information
1. What is Tabrecta and what is it used for
What is Tabrecta
Tabrecta contains the active substance capmatinib, which belongs to a group of medicines called protein kinase inhibitors.
What is Tabrecta used for
Tabrecta is a medicine used to treat adult patients with a type of lung cancer called non-small cell lung cancer (NSCLC). It is used if the lung cancer is advanced or has spread to other parts of the body (metastatic) and is caused by a change (mutation) in a gene that produces an enzyme called MET.
Tumor or blood tests will be performed to detect certain mutations in this gene. If the result is positive, it is possible that your cancer may respond to treatment with Tabrecta.
How Tabrecta works
Tabrecta helps to slow down or stop the growth and spread of lung cancer if it is caused by a mutation in a gene that produces MET.
If you have any questions about how Tabrecta works or why you have been prescribed this medicine, ask your doctor or pharmacist.
2. What you need to know before you take Tabrecta
Do not take Tabrecta
- if you are allergic to capmatinib or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Talk to your doctor, pharmacist, or nurse before taking Tabrecta:
- if you have or have had lung problems or breathing difficulties apart from lung cancer.
- if you have or have had liver problems.
- if you have or have had pancreatic problems.
While taking Tabrecta, you should limit your exposure to direct sunlight or ultraviolet (UV) artificial light. You should use sunscreen, wear sunglasses, and wear clothing to cover your skin, and you should avoid sunbathing while taking Tabrecta and for at least 7 days after stopping treatment.
Tell your doctor, pharmacist, or nurse immediately if you have an allergic reaction during treatment with Tabrecta:
- Symptoms of an allergic reaction may include rash, hives, fever, difficulty breathing, or low blood pressure.
Checks during treatment with Tabrecta
Your doctor will perform blood tests before starting treatment with Tabrecta to check your liver and pancreas function. Your doctor will continue to check your liver and pancreas function during treatment with Tabrecta.
Children and adolescents
Do not give this medicine to children and adolescents under 18 years of age, as it has not been studied in this age group.
Other medicines and Tabrecta
Tell your doctor or pharmacist if you are taking, have recently taken, or might take any other medicines.
It is particularly important that you mention any of the following medicines:
- medicines used to treat seizures, such as carbamazepine, phenobarbital, phenytoin
- St. John's Wort (also known as Hypericum perforatum), a herbal product used to treat depression and other conditions
- medicines used to treat tuberculosis, such as rifampicin
- antibiotics used to treat bacterial infections, such as telithromycin, clarithromycin
- medicines used to treat fungal infections, such as ketoconazole, itraconazole, posaconazole, voriconazole
- medicines used to treat HIV/AIDS, such as ritonavir (alone or in combination with lopinavir), saquinavir, indinavir, nelfinavir, efavirenz
- medicines used to treat hepatitis, such as telaprevir
- medicines used to treat depression, such as nefazodone
- medicines used to treat high blood pressure or heart problems, such as verapamil
- medicines used to treat respiratory problems, such as theophylline
- medicines used to treat muscle spasms, such as tizanidine
- medicines used to treat heart problems, such as digoxin
- medicines used to treat blood clots, such as dabigatran etexilate
- medicines used to treat gout, such as colchicine
- medicines used to treat diabetes, such as sitagliptin, saxagliptin
- medicines used to treat high cholesterol, such as rosuvastatin, pravastatin
- medicines used to treat certain types of cancer or autoimmune diseases, such as methotrexate, mitoxantrone
- sulfasalazine, a medicine used to treat inflammation in the intestine and rheumatic inflammation in the joints
Tell your doctor, pharmacist, or nurse if you are not sure if you are taking any of the above medicines.
You should also tell your doctor if you are prescribed a new medicine while you are on treatment with Tabrecta.
Pregnancy and breastfeeding
Tabrecta may harm your unborn baby. If you are a woman who can become pregnant, your doctor will perform a pregnancy test before you start treatment with Tabrecta to make sure you are not pregnant. You must use an effective method of contraception while you are taking Tabrecta and for at least 7 days after stopping treatment. Ask your doctor about effective methods of contraception.
If you become pregnant or think you may be pregnant while taking Tabrecta, tell your doctor immediately. Your doctor will discuss the potential risks of taking Tabrecta during pregnancy.
If you are a man with a pregnant partner or a partner who may become pregnant, you must use condoms while you are taking Tabrecta and for at least 7 days after stopping treatment.
It is not known if Tabrecta passes into breast milk. Do not breastfeed while you are taking Tabrecta and for at least 7 days after stopping treatment.
Driving and using machines
Tabrecta is not expected to affect your ability to drive or use machines.
Tabrecta contains sodium
This medicine contains less than 1 mmol of sodium (23 mg) per dose; this is essentially "sodium-free".
3. How to take Tabrecta
Take this medicine exactly as your doctor or pharmacist has told you. If you are not sure, ask your doctor or pharmacist.
Do not take more than the recommended dose that your doctor has prescribed for you.
How much Tabrecta to take
The recommended dose is 400 mg (2 tablets of 200 mg) orally twice a day, with or without food. Taking Tabrecta twice a day, at the same time each day, will help you remember when to take your medicine. If you have difficulty swallowing the tablets, take Tabrecta tablets with food.
Your doctor will tell you exactly how many Tabrecta tablets to take. Your doctor may change the dose during treatment with Tabrecta if you have some side effects. Do not change the dose without talking to your doctor.
Swallow the Tabrecta tablets whole. Do not break, crush, or chew the tablets.
If you vomit after taking Tabrecta, do not take any more Tabrecta tablets until it is time for your next dose.
How long to take Tabrecta
Keep taking Tabrecta for as long as your doctor tells you.
This is a long-term treatment, which may last for months or years. Your doctor will monitor your condition to check that the treatment is having the desired effect.
If you have questions about how long to take Tabrecta, ask your doctor or pharmacist.
If you take more Tabrecta than you should
If you have taken too many Tabrecta tablets, or if someone else has taken your medicine by mistake, contact your doctor or hospital immediately. You should show them the pack of Tabrecta. Medical treatment may be necessary.
If you forget to take Tabrecta
Do not take a double dose to make up for forgotten doses. Wait until it is time for your next dose.
If you stop taking Tabrecta
Your doctor may stop your treatment with Tabrecta temporarily or permanently if you have some side effects. Do not stop taking your medicine unless your doctor tells you to.
If you have any other questions about the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Some side effects may be serious
If you get any of the serious side effects listed below, tell your doctor immediately. Your doctor may advise you to stop taking the medicine or change the dose.
Very common:may affect more than 1 in 10 people
- Abnormal blood test results, such as high levels of alanine aminotransferase (ALT) and/or aspartate aminotransferase (AST), which may be a sign of liver problems
- Abnormal blood test results, such as high levels of amylase and/or lipase, which may be a sign of pancreatic problems
Common:may affect up to 1 in 10 people
- Abnormal blood test results, such as high levels of bilirubin, which may be a sign of liver problems
- Cough, fever, breathing difficulties, shortness of breath, or wheezing, which may be a sign of inflammation of the lungs (pneumonitis, interstitial lung disease)
- Urinating less often than usual or with less urine than normal, which may be a sign of kidney problems (renal failure, acute kidney injury)
Uncommon:may affect up to 1 in 100 people
- Severe pain in the upper abdomen, which may be a sign of inflammation of the pancreas (acute pancreatitis)
- Allergic reaction (hypersensitivity), which may include rash, hives, fever, difficulty breathing, or low blood pressure
Other possible side effects
Other side effects include the following list. If these side effects become serious, tell your doctor, pharmacist, or nurse.
Very common:may affect more than 1 in 10 people
- Swelling in the hands, elbows, or feet (peripheral edema)
- Nausea and/or vomiting
- Fatigue and/or weakness (fatigue, asthenia)
- Shortness of breath (dyspnea)
- Loss of appetite
- Back pain
- Cough
- Changes in bowel movements (diarrhea or constipation)
- Weight loss
- Fever (pyrexia)
- Itching without rash (pruritus)
- Rash
Common:may affect up to 1 in 10 people
- Chest pain
- Pain, tenderness, redness, warmth, or swelling of the skin, which may be a sign of bacterial infection of the skin (cellulitis)
- Itching with rash (urticaria)
Abnormal blood test results
During treatment with Tabrecta, blood test results may be abnormal, which may be a sign of kidney, liver, or electrolyte problems. These include:
Very common:may affect more than 1 in 10 people
- Low albumin levels in the blood
- High creatinine levels in the blood (a substance excreted by the kidneys)
- Low phosphate levels in the blood
- Low sodium levels in the blood
Reporting of side effects
If you experience any side effects, talk to your doctor, pharmacist, or nurse, even if they are not listed in this leaflet. You can also report side effects directly through the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Tabrecta
- Keep this medicine out of the sight and reach of children.
- Do not use this medicine after the expiry date which is stated on the carton after CAD and on the blister after EXP. The expiry date is the last day of the month shown.
- This medicine does not require any special storage conditions. Store in the original package to protect from moisture.
- Do not use this medicine if you notice any damage to the packaging or if it shows signs of tampering.
- Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Container Content and Additional Information
Composition of Tabrecta
- The active ingredient is capmatinib.
- Each 150 mg film-coated tablet contains capmatinib dihydrochloride monohydrate equivalent to 150 mg of capmatinib.
- Each 200 mg film-coated tablet contains capmatinib dihydrochloride monohydrate equivalent to 200 mg of capmatinib.
- The other ingredients are:
- Core of the tablet: microcrystalline cellulose; mannitol; crospovidone; povidone; magnesium stearate; anhydrous colloidal silica; sodium lauryl sulfate (see "Tabrecta contains sodium" in section 2).
- Coating (150 mg): hypromellose; titanium dioxide (E171); macrogol; talc; yellow iron oxide (E172); red iron oxide (E172); black iron oxide (E172).
- Coating (200 mg): hypromellose; titanium dioxide (E171); macrogol; talc; yellow iron oxide (E172).
Appearance of Tabrecta and Container Content
Tabrecta 150 mg film-coated tablets (tablets) are oval-shaped, light orange-brown tablets. They have the marking “DU” on one side and “NVR” on the other side. Approximate size: 18.3 mm (length) x 7.3 mm (width).
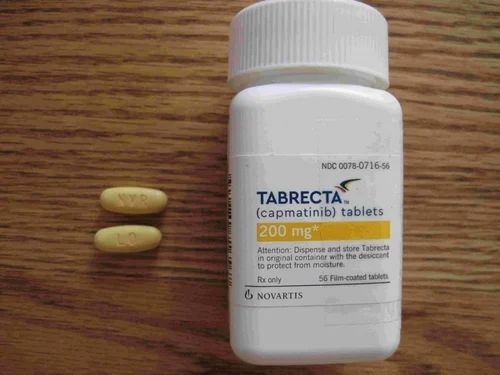
Tabrecta 200 mg film-coated tablets (tablets) are oval-shaped, yellow tablets. They have the marking “LO” on one side and “NVR” on the other side. Approximate size: 20.3 mm (length) x 8.1 mm (width).
Tabrecta film-coated tablets are presented in blisters and are available in packs containing 60 or 120 film-coated tablets.
Not all pack sizes may be marketed.
Marketing Authorization Holder
Novartis Europharm Limited
Vista Building
Elm Park, Merrion Road
Dublin 4
Ireland
Manufacturer
Lek Pharmaceuticals d.d.
Trimlini 2D
9220 Lendava
Slovenia
Novartis Pharma GmbH
Sophie-Germain-Strasse 10
90443 Nürnberg
Germany
Novartis Farmacéutica S.A.
Gran Via de les Corts Catalanes, 764
08013 Barcelona
Spain
You can request more information about this medicinal product by contacting the local representative of the marketing authorization holder:
België/Belgique/Belgien Novartis Pharma N.V. Tel: +32 2 246 16 11 | Lietuva SIA Novartis Baltics Lietuvos filialas Tel: +370 5 269 16 50 |
Novartis Bulgaria EOOD Tel: +359 2 489 98 28 | Luxembourg/Luxemburg Novartis Pharma N.V. Tel: +32 2 246 16 11 |
Ceská republika Novartis s.r.o. Tel: +420 225 775 111 | Magyarország Novartis Hungária Kft. Tel.: +36 1 457 65 00 |
Danmark Novartis Healthcare A/S Tlf.: +45 39 16 84 00 | Malta Novartis Pharma Services Inc. Tel: +356 2122 2872 |
Deutschland Novartis Pharma GmbH Tel: +49 911 273 0 | Nederland Novartis Pharma B.V. Tel: +31 88 04 52 111 |
Eesti SIA Novartis Baltics Eesti filiaal Tel: +372 66 30 810 | Norge Novartis Norge AS Tlf: +47 23 05 20 00 |
Ελλáδα Novartis (Hellas) A.E.B.E. Τηλ: +30 210 281 17 12 | Österreich Novartis Pharma GmbH Tel: +43 1 86 6570 |
España Novartis Farmacéutica, S.A. Tel: +34 93 306 42 00 | Polska Novartis Poland Sp. z o.o. Tel.: +48 22 375 4888 |
France Novartis Pharma S.A.S. Tél: +33 1 55 47 66 00 | Portugal Novartis Farma - Produtos Farmacêuticos, S.A. Tel: +351 21 000 8600 |
Hrvatska Novartis Hrvatska d.o.o. Tel. +385 1 6274 220 | România Novartis Pharma Services Romania SRL Tel: +40 21 31299 01 |
Ireland Novartis Ireland Limited Tel: +353 1 260 12 55 | Slovenija Novartis Pharma Services Inc. Tel: +386 1 300 75 50 |
Ísland Vistor hf. Sími: +354 535 7000 | Slovenská republika Novartis Slovakia s.r.o. Tel: +421 2 5542 5439 |
Italia Novartis Farma S.p.A. Tel: +39 02 96 54 1 | Suomi/Finland Novartis Finland Oy Puh/Tel: +358 (0)10 6133 200 |
Κúπρος Novartis Pharma Services Inc. Τηλ: +357 22 690 690 | Sverige Novartis Sverige AB Tel: +46 8 732 32 00 |
Latvija SIA Novartis Baltics Tel: +371 67 887 070 |
Date of Last Revision of this Leaflet
Other Sources of Information
Detailed information on this medicinal product is available on the European Medicines Agency website: http://www.ema.europa.eu
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to TABRECTA 200 mg FILM-COATED TABLETSDosage form: TABLET, 150 mgActive substance: capmatinibManufacturer: Novartis Europharm LimitedPrescription requiredDosage form: TABLET, 100 mgActive substance: avapritinibManufacturer: Blueprint Medicines (Netherlands) B.V.Prescription requiredDosage form: TABLET, 200 mgActive substance: avapritinibManufacturer: Blueprint Medicines (Netherlands) B.V.Prescription required
Online doctors for TABRECTA 200 mg FILM-COATED TABLETS
Discuss questions about TABRECTA 200 mg FILM-COATED TABLETS, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions






