
MAGNESIUM SULFATE BASE 500 MG/ML INJECTABLE SOLUTION AND PERFUSION SOLUTION

How to use MAGNESIUM SULFATE BASE 500 MG/ML INJECTABLE SOLUTION AND PERFUSION SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Magnesium Sulfate Basi 500 mg/ml Solution for Injection and Infusion
magnesium sulfate heptahydrate
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only, and you should not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Magnesium Sulfate Basi and what is it used for
- What you need to know before you are given Magnesium Sulfate Basi
- How Magnesium Sulfate Basi is given
- Possible side effects
- Storage of Magnesium Sulfate Basi
- Contents of the pack and further information
1. What is Magnesium Sulfate Basi and what is it used for
Magnesium Sulfate Basi contains magnesium (as magnesium sulfate heptahydrate). Magnesium sulfate heptahydrate (hereafter referred to as magnesium sulfate) is a magnesium salt. It is used:
- to treat magnesium deficiency;
- to prevent and treat low magnesium levels in the blood in patients receiving total parenteral nutrition (nutrients are infused into the bloodstream);
- to treat a heart rhythm disorder known as "torsade de pointes";
- to control and prevent seizures in severe pre-eclampsia (a serious complication of pregnancy characterized by high blood pressure and protein in the urine);
- to control and prevent recurrent seizures in eclampsia (seizures resulting from pre-eclampsia).
The medicine is intended for use in adults, adolescents, and children.
2. What you need to know before you are given Magnesium Sulfate Basi
You should not be given Magnesium Sulfate Basi
- if you are allergic to magnesium sulfate, its salts, or any of the other ingredients of this medicine (listed in section 6).
- if you have high magnesium levels in the blood;
- if you have severe kidney impairment or renal failure (if dialysis or other blood purification methods are not available).
If any of the above applies to you, tell your doctor or nurse before you are given magnesium sulfate.
Warnings and precautions
Talk to your doctor, pharmacist, or nurse before you are given magnesium sulfate:
- if you have a disease that causes muscle weakness and fatigue called "myasthenia gravis";
- if you have kidney problems (you may need a reduced dose);
- if you have a tendency to develop kidney stones (diathesis of calcium, magnesium, ammonium, or phosphate stones);
- if you have liver problems;
- if you have heart problems.
Too rapid administration can cause a rapidly developing dilation of blood vessels and a reduction in blood pressure.
Tell your doctor or nurse if you experience flushing and sweating.
Pain, redness, swelling, or warmth at the injection site, drainage at the injection site, prolonged bleeding, cellulitis, sterile abscess, signs of an allergic reaction, such as difficulty breathing or facial swelling, injury to nearby structures (blood vessels, bones, or nerves), accidental injection into a blood vessel, tissue necrosis, and poor absorption due to high injection volume relative to magnesium sulfate injections.
As with all parenteral medicines, this medicine may irritate the veins; leakage of the medicine from a blood vessel into the surrounding tissue can cause tissue damage.
During treatment, your blood magnesium and calcium levels will be monitored.
Your reflexes, breathing, and urine production will also be monitored while you are receiving magnesium sulfate.
Other medicines and Magnesium Sulfate Basi
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicines.
Medicines that may interact with magnesium sulfate include:
- Muscle relaxants, e.g., vecuronium
- Nifedipine (used to treat high blood pressure or chest pain)
- Calcium channel blockers (medicines to treat high blood pressure and chest pain)
- Diuretics (medicines that increase urine production) such as thiazides and furosemide
- Calcium salts
- Digitalis glycosides, e.g., digoxin (a medicine used to treat heart problems)
- Neuromuscular blocking agents
- Aminoglycoside antibacterial agents (medicines used to treat bacterial infections)
- Barbiturates (medicines to treat anxiety, insomnia)
- Opioids (medicines to treat chronic pain) such as morphine
- Hypnotics (medicines for sleep disorders)
Pregnancy, breast-feeding, and fertility
If you are pregnant or breast-feeding, think you may be pregnant, or are planning to have a baby, ask your doctor for advice before using this medicine.
Magnesium sulfate can be used to treat seizures associated with pre-eclampsia and eclampsia, serious complications of pregnancy. If you are pregnant and are given magnesium sulfate, your baby's heart rate will be closely monitored, and its use will be avoided in the 2 hours before delivery.
Magnesium sulfate has no effect on fertility.
Driving and using machines
It is unlikely that magnesium sulfate will affect your ability to drive or use machines. However, some people may feel dizzy or drowsy after receiving a magnesium sulfate injection. If you experience these side effects, do not drive or use machines.
3. How Magnesium Sulfate Basi is given
You will be given magnesium sulfate into a muscle or into a vein by slow injection or infusion.
Your doctor will decide how much magnesium sulfate you should be given. The dose depends on your individual needs and response to treatment.
Adults
Treatment of magnesium deficiency
The usual dose is 8-12 g of magnesium sulfate (32.8-49.2 mmol) in the first 24 hours, followed by 4-6 g/day (16.4-24.6 mmol/day) for 3 or 4 days, to replenish body stores.
In patients receiving total parenteral nutrition, the dose is strictly individual. As a general recommendation, 1-3 g/day (4.1-12.3 mmol/day) is administered into a vein.
Prevention and control of seizures in severe pre-eclampsia and eclampsia
An initial loading dose of 4 g of magnesium sulfate (16.4 mmol), diluted to an appropriate volume, administered into a vein, followed by an intravenous infusion of 1-2 g/hour or regular intramuscular injections, until seizures cease.
Torsade de pointes
A single dose of 2 g (8.2 mmol) administered over 2-3 minutes. An intravenous infusion is started at a rate of 2-4 mg/min.
If torsade de pointes recurs, another 2 g is administered, and the infusion rate is increased to 6-8 mg/min.
Patients with kidney problems
A reduced dose will usually be given to patients with kidney problems.
Patients with liver problems
There are no specific dose recommendations.
Elderly patients
There are no specific dose recommendations. However, caution should be exercised, as renal and/or hepatic disorders are more frequent in this age group, and adverse effects are more likely to occur.
Use in children
In children, magnesium sulfate can be administered into a vein to replenish body stores. In children receiving total parenteral nutrition, the dose is adjusted according to age, body weight, and individual needs.
If you are given too much Magnesium Sulfate Basi
Since the administration of this medicine will be carried out by a doctor or nurse, it is unlikely that you will be given too much. However, tell your doctor or nurse if you have any concerns.
In case of overdose or accidental ingestion, consult your doctor or pharmacist immediately or call the Toxicology Information Service, telephone 91 562 04 20, indicating the medicine and the amount ingested.
If you miss a dose of Magnesium Sulfate Basi
It is unlikely that you will miss a dose, as it will be given to you by your doctor or nurse. You should not receive a double dose to make up for a missed dose. Ask your doctor or nurse when you should receive the next dose.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Frequency not known(cannot be estimated from the available data)
- Allergic reactions
- High magnesium levels in the blood
- Electrolyte/fluid disturbances
- Breathing difficulties
- Feeling or being sick
- Drowsiness
- Confusion
- Slurred speech
- Double vision
- Loss of tendon reflexes
- Irregular heartbeats
- Cardiac arrest
- Abnormal electrocardiogram
- Slow heart rate
- Redness of the skin and low blood pressure due to dilation of blood vessels
- Muscle weakness
- Thirst
- Coma
Low calcium levels in the blood have been reported in pregnant women and their developing babies in extremely rare cases with high doses of magnesium sulfate.
Reporting of side effects
If you experience any side effects, talk to your doctor, pharmacist, or nurse, even if it is possible side effects not listed in this leaflet. You can also report side effects directly through the national reporting system: www.notificaRAM.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Magnesium Sulfate Basi
Keep this medicine out of the sight and reach of children.
Store below 25°C.
For single use. If only part of an ampoule is used, the remaining solution should be discarded.
Do not use this medicine after the expiry date which is stated on the label and carton after EXP. The expiry date is the last day of the month stated.
Do not use this medicine if you notice visible signs of deterioration (e.g., particles).
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Container Content and Additional Information
Composition of Magnesium Sulfate Basi 500 mg/ml injectable and perfusion solution
- The active ingredient is magnesium sulfate heptahydrate.
Each 1 ml of solution contains 500 mg of magnesium sulfate heptahydrate.
Each 10 ml ampoule contains 5,000 mg of magnesium sulfate heptahydrate.
- The other component (excipient) is water for injectable preparations.
Product Appearance and Container Content
Clear, colorless or almost colorless solution, free from visible particles.
10 ml of solution in transparent glass ampoules type I with break point.
Container size:
10 ampoules.
Only some container sizes may be marketed.
Marketing Authorization Holder and Manufacturer
Marketing Authorization Holder
Laboratórios Basi – Indústria Farmacêutica, S.A.
Parque Industrial Manuel Lourenço Ferreira, Lote 15
3450-232 Mortágua
Portugal
Tel.: + 351 231 920 250 | Fax: + 351 231 921 055
E-mail: [email protected]
Manufacturer
Laboratórios Basi - Indústria Farmacêutica, S.A.
Parque Industrial Manuel Lourenço Ferreira, Lotes 8, 15, 16
3450-232 Mortágua
Portugal
Local Representative
Laphysan, S.A.U.
Calle Anabel Segura 11,
Complejo Empresarial Albatros, Edificio A, Planta 4, puerta D,
28108 Alcobendas (Madrid) España
This medicinal product is authorized in the Member States of the European Economic Areawith the following names:
Portugal Magnesium Sulfate Basi
Spain Magnesium Sulfate Basi 500 mg/ml injectable and perfusion solution
Date of the last revision of this leaflet:April 2024
Detailed information on this medicinal product is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS): http://www.aemps.gob.es/
------------------------------------------------------------------------------------------------------------------------
This information is intended exclusively for healthcare professionals:
Posology
1 g of magnesium sulfate heptahydrate = 98.6 mg or 8.1 mEq or 4.1 mmol of magnesium.
Adults
Hypomagnesemia
The dose is strictly individual. As a general guideline, 8-12 g of magnesium sulfate (32.8-49.2 mmol) can be administered in the first 24 hours, followed by 4-6 g/day (16.4-24.6 mmol/day) for 3 or 4 days, to replenish body stores.
The maximum perfusion rates should not exceed 2 g/h. The goal should be to maintain serum magnesium concentrations above 0.4 mmol/l.
Prevention and treatment of hypomagnesemia in total parenteral nutrition
The dose is strictly individual. As a general recommendation, 1-3 g/day of magnesium sulfate can be administered intravenously (4.1-12.3 mmol/day).
Severe preeclampsia or eclampsia
By intravenous route, an initial loading dose of 4 g diluted to an appropriate volume, for example, 4 g of magnesium sulfate (16.4 mmol) in 250 ml of 5% glucose solution or 0.9% sodium chloride solution at a maximum of 4 ml/min (= 64 mg/min) can be perfused. This is followed by a maintenance regimen of intravenous perfusion of 1-2 g/hour, or regular intramuscular injections, depending on the continued presence of the patellar reflex and adequate respiratory and urinary function. Treatment should continue until seizures cease.
It is essential that when administering magnesium sulfate through any of these schemes, certain clinical observations should be made before each injection:
- deep tendon reflexes should be present;
- respiration should be at least 16 breaths/minute;
- at least 100 ml of urine should have been excreted since the previous injection.
Additionally, 1 g of calcium gluconate should be available as an antidote for hypermagnesemia.
Torsade de pointes
As a general recommendation, a single intravenous bolus of 2 g (8.2 mmol) can be administered over a period of 10 to 15 minutes. An intravenous magnesium perfusion should be started at a rate of 2-4 mg/min. If torsade de pointes recurs, another bolus of 2 g of magnesium should be administered and the intravenous perfusion rate should be increased to 6-8 mg/min. A third bolus of 2 g (8.2 mmol) is rarely required.
Pediatric Population
Hypomagnesemia
Magnesium Sulfate Basi can be administered intravenously to children. For intravenous use in children, the administration rate should not exceed 10 mg/kg/minute of magnesium sulfate (corresponding to 0.04 mmol/kg/minute = 0.001 g/kg/minute of magnesium).
Prevention and treatment of hypomagnesemia in total parenteral nutrition
The dose is strictly individual. As a general recommendation, the following doses of magnesium sulfate can be administered intravenously:
Age | Preterm infants during the first days of life | Premature infants in growth | 0-6 months | 7-12 months | 1-18 years |
Magnesium (mg/kg/day) | 2.5-5 | 5-7.5 | 2.4-5 | 4 | 2.4 |
Magnesium (mmol/kg/day) | 0.01-0.02 | 0.02-0.031 | 0.0098-0.02 | 0.0098 | 0.016 |
Renal Insufficiency
Patients with renal insufficiency should receive 25-50% of the initial recommended dose for patients with normal renal function. ECG monitoring is recommended with high doses and in elderly patients.
Hepatic Impairment
Due to insufficient data, no special dosage instructions are recommended for patients with impaired hepatic function.
Elderly
Parenteral magnesium sulfate should be used with caution in elderly patients, as renal disorders are more frequent in this age group and tolerance to adverse effects may be lower.
Administration Route
For intravenous (injection or perfusion) or intramuscular use, according to the information provided for each indication.
The medication should be administered with caution if redness and sweating occur.
Too rapid administration can cause rapid development of vasodilation and reduction of blood pressure.
As with all parenteral medications, magnesium sulfate injections can irritate veins; extravasation can cause tissue damage.
The medication should not be administered in demarcated or atrophied muscles. For intramuscular administration, the dorsogluteal muscle and the sciatic nerve should be avoided. If the total dose to be administered exceeds 5 ml, the injection volume should be divided between more than one deep muscle injection site.
Intramuscular injections are painful and complicated by the formation of local abscesses in 0.5% of cases. Therefore, the intravenous route is preferred. However, the intramuscular regimen becomes the best option when intravenous perfusion pumps are not available or continuous monitoring is not feasible.
Use with caution in elderly or thin patients who can only tolerate up to 2 ml in a single injection. Do not use an injection site with evidence of infection or injury. If repeating an intramuscular dose, alternate injection sites to avoid muscle injury or discomfort.
Instructions for Use, Disposal, and Other Handling
For single use.
Can be diluted with 0.9% sodium chloride solution and 5% glucose solution.
The medication should be used immediately after opening the ampoule. Any unused portion should be discarded.
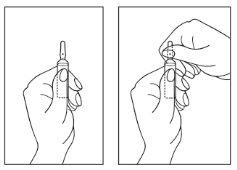
Instructions for opening the ampoule
- Turn the ampoule with the colored point upwards. If there is solution in the upper part of the ampoule, gently tap with your finger to ensure all the solution reaches the lower part of the ampoule.
- Use both hands to open; while holding the lower part of the ampoule with one hand, use the other to separate the upper part of the ampoule from the colored point (see images below).
1 g of calcium gluconate solution should be available as an antidote.
Disposal of unused medication and all materials that have come into contact with it will be carried out in accordance with local regulations.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to MAGNESIUM SULFATE BASE 500 MG/ML INJECTABLE SOLUTION AND PERFUSION SOLUTIONDosage form: INJECTABLE, 150 mg/mlActive substance: magnesium sulfateManufacturer: Altan Pharmaceuticals SaPrescription requiredDosage form: INJECTABLE, 200 mg/mLActive substance: magnesium sulfateManufacturer: Laboratorios Basi Industria Farmaceutica S.A.Prescription requiredDosage form: INJECTABLE, 1500 mgActive substance: magnesium sulfateManufacturer: Desma Laboratorio Farmaceutico S.L.Prescription required
Online doctors for MAGNESIUM SULFATE BASE 500 MG/ML INJECTABLE SOLUTION AND PERFUSION SOLUTION
Discuss questions about MAGNESIUM SULFATE BASE 500 MG/ML INJECTABLE SOLUTION AND PERFUSION SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















