STRENSIQ 40 mg/mL injectable solution

How to use STRENSIQ 40 mg/mL injectable solution
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Strensiq 40 mg/ml Solution for Injection
(12 mg/0.3 ml, 18 mg/0.45 ml, 28 mg/0.7 ml, 40 mg/1 ml)
asfotase alfa
This medicine is subject to additional monitoring, which will help to quickly identify new safety information. You can help by reporting any side effects you may get. The last section of this leaflet includes information on how to report side effects.
Read all of this leaflet carefully before you start using this medicine, because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the Package Leaflet
- What is Strensiq and what is it used for
- What you need to know before you use Strensiq
- How to use Strensiq
- Possible side effects
- Storing Strensiq
- Contents of the pack and other information
1. What is Strensiq and what is it used for
What is Strensiq
Strensiq is a medicine used to treat a rare inherited disease called hypophosphatasia that started in childhood. It contains the active substance asfotase alfa.
What is hypophosphatasia
Patients with hypophosphatasia have low levels of an enzyme called alkaline phosphatase, which is important for various bodily functions, including the proper strengthening of bones and teeth. Patients have bone growth and strength problems that can lead to bone fractures, bone pain, and difficulty walking, as well as breathing difficulties and a risk of seizures (epileptic fits).
What is Strensiq used for
The active substance in Strensiq can replace the missing enzyme (alkaline phosphatase) in hypophosphatasia. It is used as prolonged enzyme replacement therapy to treat the symptoms.
What benefits has Strensiq shown in clinical studies
Strensiq has shown benefits in bone mineralization and patient growth.
1
2. What you need to know before you use Strensiq
Do not use Strensiq
If you are severely allergic to asfotase alfa (see section "Warnings and precautions" below) or to any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Consult your doctor before using Strensiq.
- Patient treated with asfotase alfa have presented allergic reactions, including potentially life-threatening allergic reactions similar to anaphylaxis that required medical treatment. Patients who presented symptoms similar to anaphylaxis had difficulty breathing, feeling of suffocation, nausea, swelling around the eyes, and dizziness. Reactions occurred within minutes of asfotase alfa administration and can occur in patients who have been taking asfotase alfa for more than a year. If you experience any of these symptoms, stop Strensiq and seek medical attention immediately.
If you experience an anaphylactic reaction or an episode with similar symptoms, your doctor will discuss the measures to be taken next and the possibility of resuming Strensiq under medical supervision. Always follow the instructions provided by your doctor.
- During treatment, blood proteins against Strensiq, also called antibodies against the medicine, may develop. Inform your doctor if you experience reduced efficacy of Strensiq.
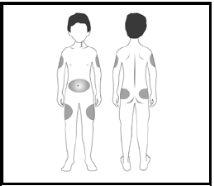
- Fat lumps or reduced fat tissue on the skin surface (localized lipodystrophy) at the injection sites have been reported after several months of treatment with Strensiq. Read section 3 carefully for injection recommendations. It is essential to change the injection site between the following body areas to reduce the risk of lipodystrophy: abdominal area, thigh, or deltoid.
- In studies, some eye-related side effects (e.g., calcium accumulation in the eye [conjunctival or corneal calcification]) have been reported in patients using Strensiq and those who did not, probably associated with hypophosphatasia. Inform your doctor if you experience vision problems.
- Premature fusion of the skull bones (craneosinostosis) has been reported in children under 5 years of age in clinical studies of infants with hypophosphatasia, with and without the use of Strensiq. Inform your doctor if you notice any change in the shape of your child's head.
- If you receive treatment with Strensiq, you may experience an injection site reaction (pain, nodule, skin rash, discoloration) during or after the injection of the medicine. If you experience any severe injection site reaction, inform your doctor immediately.
- In studies, an increase in parathyroid hormone concentration and low calcium levels have been reported. Therefore, your doctor may ask you to take oral calcium and vitamin D supplements if necessary.
- Weight gain may occur during your treatment with Strensiq. Your doctor will provide dietary advice as needed.
Other medicines and Strensiq
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicines.
If you need to have blood tests, inform your doctor that you are receiving treatment with Strensiq. Strensiq may affect the results of some blood tests. Therefore, if you are treated with Strensiq, you may need to have other types of tests.
Pregnancy
Strensiq must not be used during pregnancy. Effective contraceptive methods should be considered during treatment in women of childbearing age.
Breast-feeding
It is not known whether Strensiq can be excreted in breast milk. Consult your doctor if you are breast-feeding or plan to breast-feed. Your doctor will then help you decide whether to stop breast-feeding or stop treatment with Strensiq, taking into account the benefit of breast-feeding for the child and the benefit of treatment with Strensiq for the mother.
If you are pregnant or breast-feeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medicine.
Driving and using machines
This medicine is not expected to affect your ability to drive or use machines.
Important information about some of the ingredients of Strensiq
This medicine contains less than 1 mmol of sodium (23 mg) per vial, which means it can be considered essentially sodium-free.
3. How to use Strensiq
Follow the administration instructions for the medication contained in this prospectus or as indicated by your doctor, pharmacist, or nurse. In case of doubt, ask your doctor, pharmacist, or nurse.
A doctor with experience in treating patients with metabolic or bone diseases will explain how to use Strensiq. After the doctor or specialized nurse has trained you, you can administer Strensiq at home.
Dose
- The dose you receive depends on your body weight.
- Your doctor will calculate the correct dose, which consists of a total of 6 mg of asfotase alfa per kg of body weight per week, administered as an injection of 1 mg/kg of asfotase alfa 6 times a week or as an injection of 2 mg/kg of asfotase alfa 3 times a week, depending on your doctor's recommendation. Each dose will be administered by subcutaneous injection (under the skin) (see the included dosage table for detailed information on the volume to be injected and the type of vials to use based on your weight).
- Your doctor should periodically adjust the dose as your weight changes.
- The maximum volume per injection should not exceed 1 ml. If more than 1 ml needs to be administered, you should perform several injections in a row, one after the other.
In case of injection 3 times a week In case of injection 6 times a week
Body weight (kg) | Volume to be injected | Color code of the vial to be used | Body weight (kg) | Volume to be injected | Color code of the vial to be used | |
3 | 0.15 ml | Dark blue | 6 | 0.15 ml | Dark blue | |
4 | 0.20 ml | Dark blue | 7 | 0.18 ml | Dark blue | |
5 | 0.25 ml | Dark blue | 8 | 0.20 ml | Dark blue | |
6 | 0.30 ml | Dark blue | 9 | 0.23 ml | Dark blue | |
7 | 0.35 ml | Orange | 10 | 0.25 ml | Dark blue | |
8 | 0.40 ml | Orange | 11 | 0.28 ml | Dark blue | |
9 | 0.45 ml | Orange | 12 | 0.30 ml | Dark blue | |
10 | 0.50 ml | Light blue | 13 | 0.33 ml | Orange | |
11 | 0.55 ml | Light blue | 14 | 0.35 ml | Orange | |
12 | 0.60 ml | Light blue | 15 | 0.38 ml | Orange | |
13 | 0.65 ml | Light blue | 16 | 0.40 ml | Orange | |
14 | 0.70 ml | Light blue | 17 | 0.43 ml | Orange | |
15 | 0.75 ml | Pink | 18 | 0.45 ml | Orange | |
16 | 0.80 ml | Pink | 19 | 0.48 ml | Light blue | |
17 | 0.85 ml | Pink | 20 | 0.50 ml | Light blue | |
18 | 0.90 ml | Pink | 25 | 0.63 ml | Light blue | |
19 | 0.95 ml | Pink | 30 | 0.75 ml | Pink | |
20 | 1 ml | Pink | 35 | 0.88 ml | Pink | |
25 | 0.50 ml | Green | 40 | 1 ml | Pink | |
30 | 0.60 ml | Green | 50 | 0.50 ml | Green | |
35 | 0.70 ml | Green | 60 | 0.60 ml | Green | |
40 | 0.80 ml | Green | 70 | 0.70 ml | Green | |
80 | 0.80 ml | Green | ||||
90 | 0.90 ml | Green (x2) | ||||
100 | 1 ml | Green (x2) |
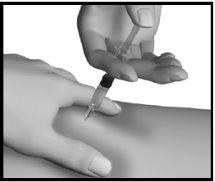
Injection recommendations
- You may experience a reaction at the injection site. Read section 4 carefully before using this medication to know the adverse effects that may occur.
- When injecting regularly, you should change the injection site between different areas of the body to help reduce possible pain and irritation.
- Areas with a greater amount of fat under the skin (thighs, arms [deltoids], abdomen, and buttocks) are the most suitable areas for injection. Consult your doctor or nurse about the best places in your case.
Before injecting Strensiq, read the following instructions carefully
- Each vial is for single use and should only be pierced once. The Strensiq liquid should have a transparent, slightly opalescent, or opalescent appearance, colorless to slightly yellow, and may contain small translucent or white particles. Do not use it if the liquid is discolored or contains lumps or large particles; obtain a new vial. The disposal of unused medication and all materials that have come into contact with it will be carried out in accordance with local regulations.
- If you are going to inject the medication yourself, your doctor, pharmacist, or nurse will teach you how to prepare and inject the medication. Do not inject this medication yourself unless you have been trained and have understood the procedure.
How to inject Strensiq:
Step 1: Preparation of the Strensiq dose
- Wash your hands well with water and soap.
- Remove the Strensiq vial(s) from the refrigerator 15 to 30 minutes before administering the injection to allow the liquid to reach room temperature. Do not heat Strensiq in any other way (e.g., do not heat it in the microwave or in hot water). After removing the vial(s) from the refrigerator, Strensiq must be used within a maximum of 3 hours.
- Remove the protective cap from the Strensiq vial(s). Remove the plastic protector from the syringe you are going to use.
- Always use a new syringe with a plastic protector.
- Place a larger gauge needle (e.g., 25G) on the empty syringe, keeping the protective cap, press and turn the needle onto the syringe in a clockwise direction until it is fixed.
- Remove the plastic protector that covers the needle of the syringe. Be careful not to prick yourself with the needle.
- Pull the plunger back to allow the same amount of air to enter the syringe as your dose.
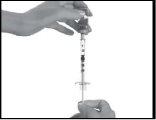
Step 2: Extraction of the Strensiq solution from the vial
|
|
|
|
|
|
- After removing the air bubbles, check the dose of medication in the syringe to ensure you have drawn the correct amount. You may need to use several vials to draw the total volume you need for the correct dose.
Step 3: Placement of the needle on the syringe
- Remove the needle from the vial. Put the cap back on with one hand by placing it on a flat surface; slide the needle into the cap, lift it, and snap it firmly into place using one hand.
- Remove the larger gauge needle by pressing and turning it counterclockwise. Place the needle with the protective cap in the sharps container.
- Place a smaller gauge needle (e.g., 27 or 29G) on the filled syringe, keeping the protective cap, press and turn the needle onto the syringe in a clockwise direction until it is fixed. Remove the protective cap from the needle.
- Hold the syringe with the needle facing upwards and gently tap the syringe cylinder with your fingers to remove any possible air bubbles.
Visually check that the volume contained in the syringe is correct.
The volume per injection should not exceed 1 ml. If you need a larger volume, you should administer several injections in different locations.
You are now ready to inject the correct dose.
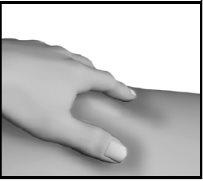
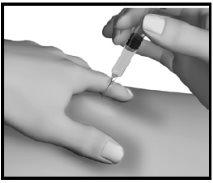
Step 4: Injection of Strensiq
|
NOTE: Do not use any area where you notice swelling, hard lumps, or pain; inform your doctor of anything you find. |
|
|
|
In patients with little fat under the skin or thin skin, a 45° angle may be preferred. |
|
This will help close the punctured tissue and prevent any loss. Do not rub the injection site after injection. |
If you need to perform a second injection to complete the prescribed dose, take another Strensiq vial and repeat steps 1 to 4.
Step 5: Disposal of used materials
Place the syringes, vials, and needles in a sharps container. Your doctor, pharmacist, or nurse will tell you how to obtain one of these containers.
If you use more Strensiq than you should
If you think you have accidentally received a higher dose of Strensiq than prescribed, contact your doctor for advice.
If you miss a dose of Strensiq
Do not inject a double dose to make up for the missed dose, and contact your doctor for advice.
If you have any other questions about the use of this medication, ask your doctor, pharmacist, or nurse.
4. Possible side effects
Like all medications, this medication can cause side effects, although not everyone will experience them.
If you are not sure what the following side effects are, ask your doctor to explain them to you.
The most serious side effects observed in patients treated with asfotase alfa have been allergic reactions, including potentially life-threatening allergic reactions similar to anaphylaxis that required medical treatment. This side effect is common (it can affect up to 1 in 10 people). Patients who experienced these severe allergic reactions had difficulty breathing, a feeling of suffocation, nausea, swelling around the eyes, and dizziness. The reactions occurred a few minutes after using asfotase alfa and can occur in patients who have been using asfotase alfa for more than a year. If you experience any of these symptoms, stop using Strensiq and seek medical attention immediately.
In addition, other allergic reactions (hypersensitivity) may occur frequently, which can manifest as redness (erythema), fever (pyrexia), skin rash, itching (pruritus), irritability, nausea, vomiting, pain, chills (shivering), numbness of the mouth (oral hypoesthesia), headache, flushing (hot flashes), rapid heart rate (tachycardia), and cough. If you experience any of these symptoms, stop using Strensiq and seek medical attention immediately.
Very common: may affect more than 1 in 10 people
Reactions at the injection site during or after injection of the medication (which can cause redness, discoloration, itching, pain, fat lumps or less fatty tissue on the skin surface, skin hypopigmentation, and/or swelling)
Fever (pyrexia)
Irritability
Redness of the skin (erythema)
Pain in the hands and feet (pain in the extremities)
Bruising (contusion)
Headache
Common: may affect up to 1 in 10 people
Stretched skin, skin discoloration
Nausea
Numbness of the mouth (oral hypoesthesia)
Muscle pain (myalgia)
Scarring
Increased tendency to bruising
Hot flashes
Skin infection at the injection site (injection site cellulitis)
Low calcium levels in the blood (hypocalcemia)
Kidney stones (nephrolithiasis)
Reporting side effects
If you experience any side effects, consult your doctor, pharmacist, or nurse, even if they are possible side effects not listed in this prospectus. You can also report them directly through the Spanish Medicines Monitoring System for Human Use: www.notificaRAM.es. By reporting side effects, you can contribute to providing more information on the safety of this medication.
5. Storage of Strensiq
Keep this medication out of the sight and reach of children.
Do not use this medication after the expiration date stated on the carton after CAD and on the vial label after EXP. The expiration date is the last day of the month indicated.
Store in a refrigerator (between 2°C and 8°C).
Do not freeze.
Store in the original packaging to protect it from light.
After opening the vial, the medication must be used immediately (within 3 hours at room temperature between 23°C and 27°C).
Medications should not be disposed of through wastewater or household waste. Ask your pharmacist how to dispose of the packaging and any unused medication. This will help protect the environment.
6. Container Content and Additional Information
Strensiq Composition
The active ingredient is asfotase alfa. Each ml of solution contains 40 mg of asfotase alfa.
Each 0.3 ml vial of solution (40 mg/ml) contains 12 mg of asfotase alfa.
Each 0.45 ml vial of solution (40 mg/ml) contains 18 mg of asfotase alfa.
Each 0.7 ml vial of solution (40 mg/ml) contains 28 mg of asfotase alfa.
Each 1 ml vial of solution (40 mg/ml) contains 40 mg of asfotase alfa.
The other components are sodium chloride, sodium phosphate monobasic monohydrate, sodium phosphate dibasic heptahydrate, and water for injectable preparations.
Product Appearance and Container Content
Strensiq is presented as a clear, slightly opalescent or opalescent, colorless to slightly yellowish aqueous injectable solution, in vials containing 0.3 ml, 0.45 ml, 0.7 ml, and 1 ml of solution. It may contain a few small translucent or white particles.
Container sizes of 1 or 12 vials.
Only some container sizes may be marketed.
Marketing Authorization Holder
Alexion Europe SAS
103-105 rue Anatole France
92300 Levallois-Perret
France
Manufacturer
Alexion Pharma International Operations Limited
College Business and Technology Park, Blanchardstown
Dublin 15
Ireland
Date of Last Revision of this Leaflet: June 2021
This medicinal product has been authorized under "exceptional circumstances". This means that due to the rarity of the disease, it has not been possible to obtain complete information on this medicinal product.
The European Medicines Agency will review any new information on this medicinal product that may become available annually, and this leaflet will be updated as necessary.
Other Sources of Information
Detailed information on this medicinal product is available on the European Medicines Agency website: http://www.ema.europa.eu. There are also links to other websites on rare diseases and orphan medicines.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to STRENSIQ 40 mg/mL injectable solutionDosage form: INJECTABLE, 100 mg/mlActive substance: asfotase alfaManufacturer: Alexion Europe SasPrescription requiredDosage form: INJECTABLE, 40 mg/mlActive substance: asfotase alfaManufacturer: Alexion Europe SasPrescription requiredDosage form: INJECTABLE, 40 mg/mlActive substance: asfotase alfaManufacturer: Alexion Europe SasPrescription required
Online doctors for STRENSIQ 40 mg/mL injectable solution
Discuss questions about STRENSIQ 40 mg/mL injectable solution, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions