SPEVIGO 150 mg SOLUTION FOR INJECTION IN PRE-FILLED SYRINGE

How to use SPEVIGO 150 mg SOLUTION FOR INJECTION IN PRE-FILLED SYRINGE
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Spevigo 150mg solution for injection in pre-filled syringe
espesolimab
This medicine is subject to additional monitoring, which will allow for quick identification of new safety information. You can help by reporting any side effects you may get. The last section of this leaflet includes information on how to report side effects.
Read all of this leaflet carefully, because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or nurse.
- If you get any side effects, talk to your doctor or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Spevigo and what is it used for
- What you need to know before you use Spevigo
- How to use Spevigo
- Possible side effects
- Storage of Spevigo
- Contents of the pack and other information
1. What is Spevigo and what is it used for
What is Spevigo
Spevigo contains the active substance espesolimab. Espesolimab belongs to a group of medicines called interleukin inhibitors (IL). This medicine works by blocking the activity of a protein called IL36R, which is involved in inflammation.
What Spevigo is used for
Spevigo is used in adults and adolescents from 12 years of age for the maintenance treatment of a rare inflammatory skin disease called generalized pustular psoriasis (GPP). During a flare, patients may have painful skin blisters that suddenly form on large areas of the skin. These blisters, also called pustules, are filled with pus. The skin may become itchy and red, dry, cracked, or scaly. Patients may also experience more general signs and symptoms, such as fever, headache, extreme fatigue, or a burning sensation on the skin.
Spevigo eliminates pustules and other skin manifestations and, consequently, may help reduce the signs and symptoms of the disease.
2. What you need to know before you use Spevigo
A doctor with experience in treating patients with inflammatory skin diseases will start and supervise your treatment.
Do not use Spevigo if:
- you are allergic to espesolimab or any of the other ingredients of this medicine (listed in section 6).
- you have active tuberculosis or other severe infections (see "Warnings and precautions").
Warnings and precautions
Talk to your doctor or nurse before starting and during treatment with Spevigo if:
- you currently have an infection or have an infection that keeps coming back. Fever, flu-like symptoms, fatigue, or difficulty breathing, cough that won't go away, hot, red, and painful skin, or a painful rash with blisters may be signs and symptoms of an infection.
- you have or have had tuberculosis or have been in close contact with someone with tuberculosis.
- you have recently received or are scheduled to receive a vaccine. You should not receive certain types of vaccines (live microorganism vaccines) for at least 16 weeks after receiving Spevigo. Your doctor will check if you need any vaccine before starting Spevigo.
- you experience symptoms such as weakness in the arms or legs that you didn't have before, or numbness (loss of sensation), tingling, or a burning sensation in any part of your body. These could be signs of peripheral neuropathy (damage to the peripheral nerves).
It is important to keep a record of the batch number of Spevigo.
Each time you get a new pack of Spevigo, write down the date and batch number (which is stated on the pack after "Batch") and keep this information in a safe place.
Infections
Tell your doctor as soon as possible if you notice any signs or symptoms of an infection while using Spevigo (see section 4 "Possible side effects").
Allergic reactions
Consult your doctor immediately if you notice any signs or symptoms of an allergic reaction during or after using this medicine. You may also have allergic reactions several days or weeks after starting Spevigo. For signs and symptoms, see section 4 "Possible side effects".
Children and adolescents
Spevigo is not recommended for use in children under 12 years of age because it has not been studied in this age group.
Other medicines and Spevigo
Tell your doctor if:
- you are taking, have recently taken, or might take any other medicines.
- you are going to receive or have recently received a vaccine. You should not receive certain types of vaccines (live microorganism vaccines) for at least 16 weeks after receiving Spevigo.
If you are not sure, consult your doctor, pharmacist, or nurse before using Spevigo and during its use.
Pregnancy and breastfeeding
Pregnancy
If you are pregnant, think you may be pregnant, or plan to become pregnant, consult your doctor before using this medicine. The reason is that it is not known how this medicine will affect your child.
Therefore, it is preferable to avoid using Spevigo during pregnancy.
If you are pregnant, you should only receive this medicine if your doctor clearly recommends it.
Breastfeeding
It is not known whether Spevigo is excreted in breast milk. Spevigo may pass into breast milk during the first few days after delivery. Therefore, you should inform your doctor if you are breastfeeding or plan to breastfeed so that you and your doctor can decide whether you can use Spevigo.
Driving and using machines
Spevigo is not expected to affect your ability to drive and use machines.
Spevigo contains polysorbate
This medicine contains 0.4 mg of polysorbate 20 in each 1 ml pre-filled syringe. Polysorbates may cause allergic reactions. Inform your doctor if you have any known allergy.
Spevigo contains sodium
This medicine contains less than 1 mmol of sodium (23 mg) per dose; this is, essentially "sodium-free".
3. How to use Spevigo
Follow exactly the administration instructions of this medicine given by your doctor or pharmacist. If you are unsure, consult your doctor or pharmacist again.
Amount of Spevigo to be used
Adults and adolescents from 12 years of age and weighing at least 40 kg
What amount? | When? | |
1.stdose | 600 mg (fourinjections of 150 mg) | When indicated by your doctor |
Following doses | 300 mg (twoinjections of 150 mg) | Every 4 weeks from the 1st dose |
The first dose will be administered by your doctor or nurse.
You and your doctor or nurse will decide if you should inject this medicine yourself. Do not inject this medicine unless your doctor or nurse has taught you how to do it. A caregiver may also administer the injections after being taught how to do it.
Read the "Instructions for use" section at the end of this leaflet before injecting Spevigo.
Adolescents from 12 years of age and weighing between 30 and less than 40 kg
What amount? | When? | |
1.stdose | 300 mg (twoinjections of 150 mg) | When indicated by your doctor |
Following doses | 150 mg (oneinjection of 150 mg) | Every 4 weeks from the 1st dose |
Spevigo will be administered by your doctor or nurse.
If you use more Spevigo than you should
If you have used more Spevigo than you should or have administered the dose before it was prescribed, consult your doctor.
If you forget to useSpevigo
If you forget to use Spevigo, inject a dose as soon as you remember. Consult your doctor if you are not sure what to do.
If you stop using Spevigo
Do not stop using Spevigo without consulting your doctor first. If you stop using Spevigo, your symptoms may come back or you may experience a flare.
If you have any other questions about the use of this medicine, ask your doctor, pharmacist, or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Consult your doctor immediately if you notice any signs or symptoms of an allergic reaction during or after using this medicine. These may be:
- difficulty breathing or swallowing
- swelling of the face, lips, tongue, or throat
- intense itching of the skin, with a red rash or different bumps from the symptoms of GPP
- feeling dizzy
You may also have allergic reactions several days or weeks after using Spevigo.
Consult your doctor immediatelyif you get a widespread skin rash, fever, and/or swelling of the face between 2 and 8 weeks after using the medicine. These could be signs of a delayed allergic reaction (hypersensitivity).
Tell your doctor as soon as possible if you notice any signs or symptoms of an infection.These may be:
Very common(may affect more than 1 in 10 people)
- fever, cough
- Common(may affect up to 1 in 10 people)
- frequent urination, pain or burning while urinating, or blood in the urine, which may be symptoms of urinary tract infections
Tell your doctor or nurse if you get any of the following other side effects:
Very common(may affect more than 1 in 10 people)
- redness, swelling, hardening, warmth, pain, scaling of the skin, small solid bumps on the skin, itching, skin rash, or hives at the injection site
Common(may affect up to 1 in 10 people)
- itching
- feeling tired
Frequency not known(cannot be estimated from the available data)
- allergic reaction
Reporting of side effects
If you experience any side effects, talk to your doctor or nurse, even if they are not listed in this leaflet. You can also report side effects directly through the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Spevigo
Keep this medicine out of the sight and reach of children.
Do notuse this medicine after the expiry date which is stated on the pre-filled syringe and the carton after EXP or CAD. The expiry date is the last day of the month stated.
Store in a refrigerator (between 2°C and 8°C). Do notfreeze. Do notuse Spevigo if it has been frozen, even if it has been thawed.
If necessary, Spevigo can be stored at temperatures up to 25°C for a maximum of 14 days. Discard Spevigo if it has been stored at temperatures up to 25°C for more than 14 days.
Store in the original package to protect from light.
Do notuse this medicine if the liquid is cloudy or contains flakes or large colored particles.
Medicines should notbe disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Container Contents and Additional Information
Composition of Spevigo
- The active ingredient is espesolimab. Each pre-filled syringe contains 150 mg of espesolimab in 1 ml of solution.
- The other components are sodium acetate trihydrate (E262), glacial acetic acid (E260) (for pH adjustment), sucrose, arginine hydrochloride, polysorbate 20 (E432), and water for injectable preparations.
Appearance of the Product and Container Contents
Spevigo injectable solution is a clear to slightly opalescent solution and colorless to a slight yellowish-brown color in a pre-filled syringe with a safety protector. The liquid may contain very small white or transparent particles. Each pre-filled syringe contains 150 mg in 1 ml of injectable solution.
Each container contains two pre-filled syringes.
Marketing Authorization Holder
Boehringer Ingelheim International GmbH
Binger Str. 173
55216 Ingelheim am Rhein
Germany
Manufacturer
Boehringer Ingelheim Pharma GmbH & Co. KG
Birkendorfer Strasse 65
88397 Biberach an der Riss
Germany
You can request more information about this medication by contacting the local representative of the marketing authorization holder:
Belgium/Belgique/Belgien Boehringer Ingelheim SComm Tel: +32 2 773 33 11 | Lithuania Boehringer Ingelheim RCV GmbH & Co KG Lithuanian branch Tel: +370 5 2595942 |
| Luxembourg/Luxemburg Boehringer Ingelheim SComm Tel: +32 2 773 33 11 |
Czech Republic Boehringer Ingelheim spol. s r.o. Tel: +420 234 655 111 | Hungary Boehringer Ingelheim RCV GmbH & Co KG Hungarian branch office Tel: +36 1 299 8900 |
Denmark Boehringer Ingelheim Danmark A/S Tel: +45 39 15 88 88 | Malta Boehringer Ingelheim Ireland Ltd. Tel: +353 1 295 9620 |
Germany Boehringer Ingelheim Pharma GmbH & Co. KG Tel: +49 (0) 800 77 90 900 | Netherlands Boehringer Ingelheim B.V. Tel: +31 (0) 800 22 55 889 |
Estonia Boehringer Ingelheim RCV GmbH & Co KG Estonian branch Tel: +372 612 8000 | Norway Boehringer Ingelheim Danmark Norwegian branch Tel: +47 66 76 13 00 |
Greece Boehringer Ingelheim Ελλάς Μονοπρόσωπη A.E. Tel: +30 2 10 89 06 300 | Austria Boehringer Ingelheim RCV GmbH & Co KG Tel: +43 1 80 105-7870 |
Spain Boehringer Ingelheim España, S.A. Tel: +34 93 404 51 00 | Poland Boehringer Ingelheim Sp. z o.o. Tel: +48 22 699 0 699 |
France Boehringer Ingelheim France S.A.S. Tel: +33 3 26 50 45 33 | Portugal Boehringer Ingelheim Portugal, Lda. Tel: +351 21 313 53 00 |
Croatia Boehringer Ingelheim Zagreb d.o.o. Tel: +385 1 2444 600 | Romania Boehringer Ingelheim RCV GmbH & Co KG Vienna - Bucharest branch Tel: +40 21 302 28 00 |
Ireland Boehringer Ingelheim Ireland Ltd. Tel: +353 1 295 9620 | Slovenia Boehringer Ingelheim RCV GmbH & Co KG Ljubljana branch office Tel: +386 1 586 40 00 |
Iceland Vistor ehf. Tel: +354 535 7000 | Slovak Republic Boehringer Ingelheim RCV GmbH & Co KG organizational unit Tel: +421 2 5810 1211 |
Italy Boehringer Ingelheim Italia S.p.A. Tel: +39 02 5355 1 | Finland Boehringer Ingelheim Finland Ky Tel: +358 10 3102 800 |
Cyprus Boehringer Ingelheim Ελλάς Μονοπρόσωπη A.E. Tel: +30 2 10 89 06 300 | Sweden Boehringer Ingelheim AB Tel: +46 8 721 21 00 |
Latvia Boehringer Ingelheim RCV GmbH & Co KG Latvian branch Tel: +371 67 240 011 |
Date of the Last Revision of this Leaflet:{MM/YYYY}.
Other Sources of Information
Detailed information on this medicinal product is available on the European Medicines Agency website: https://www.ema.europa.eu.
Instructions for Use
Spevigo150mg solution for injection in pre-filled syringe
These "Instructions for Use" contain information on how to inject Spevigo if the prescribed dose for you or your child requires 2pre-filled syringes of Spevigo 150mg.
Description of Spevigo
The pre-filled syringe contains the active ingredient espesolimab in a solution for subcutaneous injection that helps to administer a fixed dose of espesolimab.
Before starting to use this medication on yourself or your child, make sure your doctor or nurse has taught you how to use it. Then, read the leaflet and these instructions for use to ensure you administer the correct dose. If you have visual impairment and do not see well, you should be assisted by a caregiver who has been taught to use the device.
If you have any further questions, ask your doctor, pharmacist, or nurse.
Spevigo is for single use. Do notreuse the pre-filled syringe.
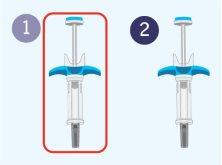
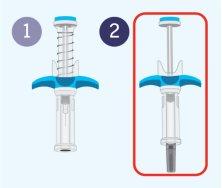
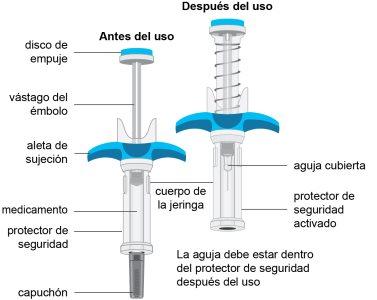
Appearance of the Spevigo pre-filled syringe
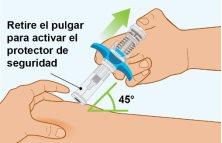
Spevigo is a pre-filled syringe with a safety protector. The needle retracts into the safety protector after injection.
The following image shows the Spevigo pre-filled syringe before use and after use with the safety protector activated.
Your doctor has prescribed a dose of Spevigo for you or your child that consists of two injections to administer a complete dose. You must inject the contents of the two pre-filled syringes that come in the box to administer the complete dose.
Importantinformation you need to know before injecting Spevigo
- Do notuse the pre-filled syringe until you have been taught the correct way to administer the injection and have read and understood these instructions for handling.
- Inspect the box that the product comes in to ensure it contains the correct medication and the correct number of pre-filled syringes for the dose prescribed for you or your child, to rule out the presence of damage, and check the expiration date.
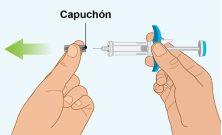
- Do notremove the cap until you are ready for injection.
- Do notuse Spevigo:
- if the liquid is cloudy or contains flakes or large particles
- if it has passed the expiration date (CAD or EXP)
- if the pre-filled syringes have been dropped or appear to be damaged
- It is important that you keep a record of the batch number of Spevigo. Each time you acquire a new package of Spevigo, note the date and batch number (which is indicated on the package after "Batch") and keep this information in a safe place.
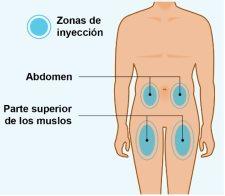
- Inject Spevigo under the skin (subcutaneous injection) in the upper part of the thighs or in the stomach area (abdomen). Do notinject Spevigo in any other area of the body.
- If you have any problems with the injection, do notproceed with the injection steps with the Spevigo pre-filled syringe. Call your doctor for help.
- If you have any further questions, ask your doctor or pharmacist.
Follow the steps indicated below when using Spevigo
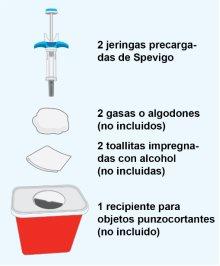
STEP1 | Preparation of Materials |
|
|
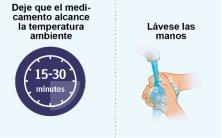
STEP2 | Preparation for Injecting Spevigo |
|
|
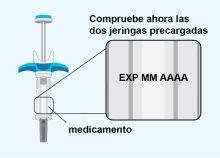
STEP3 | Inspection of the Pre-filled Syringes |
| Now check the two pre-filled syringes:
|
Preparation for the First Injection | |
| Prepare for the first of the two injections. Remember, you will repeat the following steps with the second pre-filled syringe immediately after the first injection. Two injections are needed for a complete dose. |
STEP4 | Choosing the Injection Site |
| Choose an injection site.
|
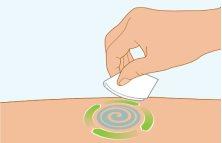
STEP5 | Cleaning the Injection Site |
|
|
STEP6 | Removing the Cap |
|
|
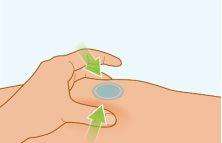
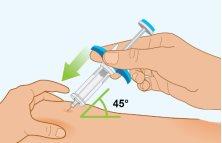
STEP7 | Pinning the Skin |
|
|
STEP8 | Before the Injection, Review Steps A, B, and C to See the Correct Way to Administer It |
Important: Do not move the pre-filled syringe when inserting the needle into the skin, during the injection, or when withdrawing the needle from the skin. | |
|
|
AInsertion of the Needle | |
| To inject Spevigo:
|
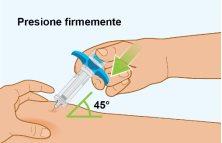
B Injection of the Medication | |
|
|
C Checking that the Injection is Complete | |
STEP9 | Second Injection |
|
The new injection site must be at a distance of at least 2 cm from the last injection site.
Important:You must inject the contents of the two Spevigo pre-filled syringes to administer a complete dose. |
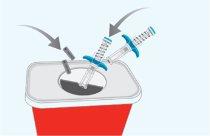
STEP10 | Disposal of Used Pre-filled Syringes and Caps |
|
|
Important:Always keep the sharps container out of the reach of children. |
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to SPEVIGO 150 mg SOLUTION FOR INJECTION IN PRE-FILLED SYRINGEDosage form: INJECTABLE PERFUSION, 450 mgActive substance: spesolimabManufacturer: Boehringer Ingelheim International GmbhPrescription requiredDosage form: INJECTABLE PERFUSION, 130 mgActive substance: ustekinumabManufacturer: Accord Healthcare S.L.U.Prescription requiredDosage form: INJECTABLE, 45 mgActive substance: ustekinumabManufacturer: Accord Healthcare S.L.U.Prescription required
Online doctors for SPEVIGO 150 mg SOLUTION FOR INJECTION IN PRE-FILLED SYRINGE
Discuss questions about SPEVIGO 150 mg SOLUTION FOR INJECTION IN PRE-FILLED SYRINGE, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions