
SIRTURO 100 MG TABLETS

How to use SIRTURO 100 MG TABLETS
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
SIRTURO 100 mg tablets
Bedaquiline
This medicine is subject to additional monitoring, which will allow for quick identification of new safety information. You can help by reporting any side effects you may get. The last section of this leaflet includes information on how to report side effects.
Read all of this leaflet carefully before you start taking this medicine, because
it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is SIRTURO and what is it used for
- What you need to know before you take SIRTURO
- How to take SIRTURO
- Possible side effects
- Storage of SIRTURO
- Contents of the pack and other information
1. What is SIRTURO and what is it used for
SIRTURO contains the active substance bedaquiline.
SIRTURO is a type of antibiotic. Antibiotics are medicines that kill bacteria that cause diseases.
SIRTURO is used to treat tuberculosis that affects the lungs when the disease has become resistant to other antibiotics. This is called multidrug-resistant pulmonary tuberculosis.
SIRTURO must always be used in combination with other medicines for the treatment of tuberculosis.
It is used in adults 18 years of age or older.
2. What you need to know before you take SIRTURO
Do not take SIRTURO:
- if you are allergic to bedaquiline or any of the other ingredients of this medicine (listed in section 6). Do not take SIRTURO if this applies to you. If you are not sure, consult your doctor or pharmacist before taking SIRTURO.
Warnings and precautions
Talk to your doctor, pharmacist, or nurse before you start taking SIRTURO, if:
- you have any abnormality in your electrocardiogram (ECG) or heart failure;
- you have a personal or family history of a heart problem called "congenital long QT syndrome";
- you have a decrease in thyroid function. This can be seen in a blood test;
- you have liver disease or if you drink alcohol regularly;
- you have a human immunodeficiency virus (HIV) infection.
If any of the above applies to you (or if you are not sure), talk to your doctor, pharmacist, or nurse before taking SIRTURO.
Children and adolescents
Do not give this medicine to children and adolescents (under 18 years of age), as it has not been studied in this age group.
Taking SIRTURO with other medicines
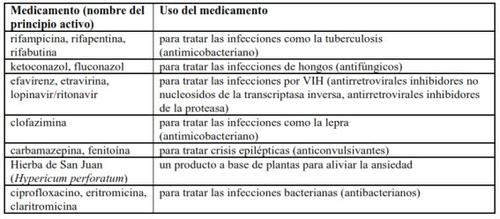
Other medicines may affect SIRTURO. Tell your doctor or pharmacist if you are taking, have recently taken, or might take any other medicines.
The following are examples of medicines that patients with multidrug-resistant tuberculosis may take and that may potentially interact with SIRTURO:
Taking SIRTURO with alcohol
Do not drink alcohol while you are taking SIRTURO.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine.
Driving and using machines
You may feel dizzy after taking SIRTURO. If this happens, do not drive or use machines.
SIRTURO contains lactose monohydrate
SIRTURO contains "lactose" (a type of sugar). If you have intolerance to some sugars or cannot digest them, talk to your doctor before taking this medicine.
3. How to take SIRTURO
Always take this medicine exactly as your doctor or pharmacist has told you. If you are not sure, check with your doctor or pharmacist.
SIRTURO must always be used in combination with other medicines for the treatment of tuberculosis. Your doctor will decide which other medicines you should take with SIRTURO.
How much to take
Take SIRTURO for a period of 24 weeks.
First 2 weeks:
- Take 400 mg (4 tablets of 100 mg) once a day.
From week 3 to week 24:
- Take 200 mg (2 tablets of 100 mg) once a day, three times a week only.
- There must be a minimum interval of 48 hours between doses when you take SIRTURO. For example, you can take SIRTURO on Mondays, Wednesdays, and Fridays every week from week 3 onwards.
You may need to continue taking your other tuberculosis medicines for more than 6 months. Ask your doctor or pharmacist.
How to take this medicine
- Take SIRTURO with food. Food is important to achieve the right levels of the medicine in your body.
- Swallow the tablets whole with water.
If you take more SIRTURO than you should
If you take more SIRTURO than you should, tell your doctor immediately. Take the medicine pack with you.
If you forget to take SIRTURO
During the first 2 weeks
- Miss the missed dose and take the next dose at the usual time.
- Do not take a double dose to make up for a missed dose.
From week 3 onwards
- Take the missed dose of 200 mg as soon as possible.
- Resume the three-times-a-week schedule.
If you are not sure what to do, talk to your doctor or pharmacist.
If you stop taking SIRTURO
Do not stop taking SIRTURO without talking to your doctor first.
Missing doses or not completing the full treatment course may:
- make the treatment ineffective and your tuberculosis worse; and
- increase the likelihood that the bacteria will become resistant to the medicine. This can make your disease not respond to treatment with SIRTURO or other medicines in the future.
If you have any further questions on the use of this medicine, ask your doctor, pharmacist, or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Very common(may affect more than 1 in 10 people):
- headache
- joint pain
- feeling dizzy
- feeling or being sick (nausea or vomiting)
Common(may affect up to 1 in 10 people):
- diarrhea
- increase in liver enzymes (seen in blood tests)
- muscle pain or tenderness not caused by exercise
- abnormality detected in the electrocardiogram called "prolongation of the QT interval". Tell your doctor immediately if you faint.
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of SIRTURO
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the carton after EXP.
The expiry date refers to the last day of the month stated.
Store SIRTURO in the original package or container to protect it from light.
This medicine may be harmful to the environment. Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Contents of the pack and other information
What SIRTURO contains
- The active substance is bedaquiline. Each tablet contains bedaquiline fumarate equivalent to 100 mg of bedaquiline.
- The other ingredients are: colloidal anhydrous silica, sodium croscarmellose, hypromellose, lactose monohydrate, magnesium stearate, corn starch, microcrystalline cellulose, polysorbate 20.
Appearance and packaging
Tablet (not coated), white to off-white, round, biconvex, 11 mm in diameter, with "T" over "207" engraved on one side and "100" on the other.
Plastic bottle with 188 tablets.
Pack containing 4 blister strips (each strip contains 6 tablets).
Not all pack sizes may be marketed.
Marketing Authorisation Holder
Janssen-Cilag International NV
Turnhoutseweg 30
B-2340 Beerse
Belgium
Manufacturer
Janssen Pharmaceutica NV
Turnhoutseweg 30
B-2340 Beerse
36
Belgium
You can get more information on this medicine by contacting the local representative of the Marketing Authorisation Holder:
België/Belgique/Belgien Janssen-Cilag NV Antwerpseweg 15-17 B-2340 Beerse Tel/Tél: +32 14 64 94 11 | Lietuva UAB "JOHNSON & JOHNSON" Konstitucijos pr. 21C LT-08130 Vilnius Tel: +370 5 278 68 88 |
???????? „??????? & ??????? ????????” ???? ?.?. ??????? 4 ?????? ???? ?????, ?????? 4 ????? 1766 ???.: +359 2 489 94 00 | Luxembourg/Luxemburg Janssen-Cilag NV Antwerpseweg 15-17 B-2340 Beerse Belgique/Belgien Tél/Tel: +32 14 64 94 11 |
Ceská republika Janssen-Cilag s.r.o. Walterovo námestí 329/1 CZ-158 00 Praha 5 – Jinonice Tel: +420 227 012 227 | Magyarország Janssen-Cilag Kft. Nagyenyed u. 8-14 H-Budapest, 1123 Tel.: +36 1 884 2858 |
Danmark Janssen-Cilag A/S Bregnerødvej 133 DK-3460 Birkerød Tlf: +45 45 94 82 82 | Malta AM MANGION LTD. Mangion Building, Triq Gdida fi Triq Valletta MT-Hal-Luqa LQA 6000 Tel: +356 2397 6000 |
Deutschland Janssen-Cilag GmbH Johnson & Johnson Platz 1 D-41470 Neuss Tel: +49 2137 955-955 | Nederland Janssen-Cilag B.V. Graaf Engelbertlaan 75 NL-4837 DS Breda Tel: +31 76 711 1111 |
Eesti UAB "JOHNSON & JOHNSON" Eesti filiaal Lõõtsa 2 EE-11415 Tallinn Tel: +372 617 7410 | Norge Janssen-Cilag AS Postboks 144 NO-1325-Lysaker Tlf: +47 24 12 65 00 |
Ελλ?da Janssen-Cilag Faρµaκeυtικ? Α.Ε.Β.Ε. Λeωf?ρος Ειρ?νης 56 GR-151 21 Πe?κη, Αθ?νa Tηλ: +30 210 80 90 000 | Österreich Janssen-Cilag Pharma GmbH Vorgartenstraße 206B A-1020 Wien Tel: +43 1 610 300 |
España Janssen-Cilag, S.A. Paseo de las Doce Estrellas, 5-7 E-28042 Madrid Tel: +34 91 722 81 00 | Polska Janssen-Cilag Polska Sp. z o.o. ul. Ilzecka 24 PL-02-135 Warszawa Tel.: +48 22 237 60 00 |
France Janssen-Cilag 1, rue Camille Desmoulins, TSA 91003 F-92787 Issy Les Moulineaux, Cedex 9 Tél: 0 800 25 50 75 / +33 1 55 00 40 03 | Portugal Janssen-Cilag Farmacêutica, Lda. Lagoas Park, Edifício 9 2740-262 PORTO SALVO PORTUGAL Tel: +351 214 368 600 |
Hrvatska Johnson & Johnson S.E. d.o.o. Oreškoviceva 6h 10010 Zagreb Tel: +385 1 6610 700 | România Johnson & Johnson România SRL Str. Tipografilor nr. 11-15 Cladirea S-Park, Corp B3-B4, Etaj 3 013714 Bucuresti, ROMÂNIA Tel: +40 21 207 1800 |
Ireland Janssen Sciences Ireland UC Barnahely Ringaskiddy IRL – Co. Cork P43 FA46 Tel: +353 1 800 709 122 | Slovenija Johnson & Johnson d.o.o. Šmartinska cesta 53 SI-1000 Ljubljana Tel: +386 1 401 18 00 |
Ísland Janssen-Cilag AB c/o Vistor hf. Hörgatúni 2 IS-210 Garðabær Sími: +354 535 7000 | Slovenská republika Johnson & Johnson s.r.o. CBC III, Karadžicova 12 SK-821 08 Bratislava Tel: +421 232 408 400 |
Italia Janssen-Cilag SpA Via M.Buonarroti, 23 I-20093 Cologno Monzese MI Tel: +39 02 2510 1 | Suomi/Finland Janssen-Cilag Oy Vaisalantie/Vaisalavägen 2 FI-02130 Espoo/Esbo Puh/Tel: +358 207 531 300 |
Κ?pρος Βaρν?ßaς Χatζηpaνaγ?ς Λtd, Λeωf?ρος Gι?ννου Κrανιdι?tη 226 Λatsι? CY-2234 Λeυκωs?a Τηλ: +357 22 207 700 | Sverige Janssen-Cilag AB Box 4042 SE-16904 Solna Tel: +46 8 626 50 00 |
Latvija UAB "JOHNSON & JOHNSON" filiale Latvija Mukusalas iela 101 Riga, LV-1004 Tel: +371 678 93561 | United Kingdom Janssen-Cilag Ltd. 50-100 Holmers Farm Way High Wycombe Buckinghamshire HP12 4EG - UK Tel: +44 1 494 567 444 |
Date of last revision of this leaflet
This medicine has been authorised with a "conditional approval".
This type of approval means that more information on this medicine is expected. The European Medicines Agency will review any new information on this medicine at least once a year and this leaflet will be updated as necessary.
Detailed information on this medicine is available on the European Medicines Agency web site: http://www.ema.europa.eu.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to SIRTURO 100 MG TABLETSDosage form: TABLET, 100 mgActive substance: bedaquilineManufacturer: Janssen-Cilag International N.VPrescription requiredDosage form: TABLET, 50 mgActive substance: delamanidManufacturer: Otsuka Novel Products GmbhPrescription requiredDosage form: TABLET, 400 mgActive substance: ethambutolManufacturer: Teofarma S.R.L.Prescription required
Online doctors for SIRTURO 100 MG TABLETS
Discuss questions about SIRTURO 100 MG TABLETS, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















