
RUBIFEN PROLONG 40 mg PROLONGED-RELEASE HARD CAPSULES

How to use RUBIFEN PROLONG 40 mg PROLONGED-RELEASE HARD CAPSULES
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Rubifen Prolong 10 mg prolonged-release hard capsules EFG
Rubifen Prolong 20 mg prolonged-release hard capsules EFG
Rubifen Prolong 30 mg prolonged-release hard capsules EFG
Rubifen Prolong 40 mg prolonged-release hard capsules EFG
Rubifen Prolong 60 mg prolonged-release hard capsules EFG
methylphenidate, hydrochloride
Read the entire package leaflet carefully before you or your child start taking this medicine, as it contains important information for you.
- Keep this package leaflet, as you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you or your child only. Do not give it to others, as it may harm them, even if their symptoms are the same as yours or your child's.
- If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this package leaflet. See section 4.
Contents of the package leaflet
- What is Rubifen Prolong and what is it used for
- What you need to know before you or your child start taking Rubifen Prolong
- How to take Rubifen Prolong
- Possible side effects
- Storage of Rubifen Prolong
- Package contents and further information
1. What is Rubifen Prolong and what is it used for
What it is used for
Methylphenidate is used to treat attention deficit hyperactivity disorder (ADHD).
- It is used in children and adolescents from 6 to 18 years old, and in adults.
- It is used only after non-medication treatments, such as counseling and behavioral therapy, have been tried first and found to be insufficient.
Methylphenidate should not be used to treat ADHD in children under 6 years old. It is not known if it is safe or effective in children under 6 years old.
How it works
Methylphenidate improves the activity of certain parts of the brain that have low activity. This medicine helps to improve attention and concentration, and reduces impulsive behavior.
This medicine is used as part of a treatment program, which usually includes:
- psychological therapy
- educational therapy and
- social therapy.
Treatment with methylphenidate should only be started and used under the supervision of a doctor specializing in the treatment of ADHD, such as a pediatrician, child and adolescent psychiatrist, or psychiatrist. A thorough examination by this doctor will be necessary. If you are an adult and have not been treated before, the specialist will carry out tests to confirm that you have had ADHD since childhood. Using treatment programs and medications helps to control ADHD.
About ADHD
Children and adolescents with ADHD may have difficulty:
- sitting still
- concentrating
It is not their fault that they cannot do these things.
Many children and adolescents try hard to do these things. However, ADHD can be problematic in daily life. Children and adolescents with ADHD may have difficulty learning and doing homework.
Adults with ADHD often have difficulty concentrating. They often feel restless, impatient, and distracted. They may have difficulty organizing their private and work life.
Not all patients with ADHD need to be treated with medication. For children, the decision to use medication should be based on a thorough assessment of the severity and chronic nature of the child's symptoms.
ADHD does not affect intelligence.
2. What you need to know before you or your child start taking Rubifen Prolong
Do not take Rubifen Prolong if you or your child:
- are allergic to methylphenidate or any of the other ingredients of this medicine (listed in section 6). If you think you may be allergic, consult your doctor
- have a thyroid problem
- have high eye pressure (glaucoma)
- have a tumor of the adrenal glands (pheochromocytoma)
- are taking a medicine called a monoamine oxidase inhibitor (MAOI) used for depression, or if you or your child have taken an MAOI in the last 14 days (see section "Other medicines and Rubifen Prolong")
- suffer from an eating disorder where you or your child do not feel hungry or do not want to eat (such as anorexia nervosa)
- have very high blood pressure or narrowing of the blood vessels, which may cause pain in the arms and legs
- have had heart problems, such as a history of heart attack, irregular heartbeat, chest pain, heart failure, heart disease, or heart problems
- have had a problem with the blood vessels in the brain, such as a stroke, swelling and weakening of part of a blood vessel (aneurysm), narrowing or blockage of blood vessels, or inflammation of the blood vessels (vasculitis)
- suffer from mental health problems, such as
- a "psychopathic personality" or "borderline personality disorder" problem
- have abnormal thoughts or hallucinations, or a disease called "schizophrenia"
- signs of a serious mood problem, such as:
- suicidal thoughts
- severe depression (feeling sad, useless, and hopeless)
- mania (unusual feeling of excitement, hyperactivity, or disinhibition)
- mood changes from depression to mania
Do not take methylphenidate if you or your child have any of the above disorders. If you are not sure, talk to your doctor or pharmacist before taking methylphenidate, as this medicine may worsen these problems.
Warnings and precautions
Talk to your doctor or pharmacist before starting to take Rubifen Prolong if you or your child:
- suffer from liver or kidney problems
- have had seizures (fits, convulsions, epilepsy) or abnormal brain tests (EEG)
- have abused or are dependent on alcohol, prescription medicines, or drugs
- are female and have started menstruating (see section "Pregnancy, breastfeeding, and fertility" later)
- or if there is a family history of repeated, uncontrollable contractions of any part of the body or repetition of sounds and words (tics)
- have high blood pressure
- suffer from a heart problem not mentioned in the section "Do not take Rubifen Prolong"
- suffer from a mental health problem not mentioned in the section "Do not take Rubifen Prolong". Other mental health problems include:
- mood changes (going from manic to depressive - called "bipolar disorder")
- experiencing aggressive or hostile behavior for the first time or worsening
- seeing, hearing, or feeling things that do not exist (hallucinations)
- believing things that are not real (delusions)
- feeling strangely suspicious (paranoia)
- feeling agitated, anxious, or tense
- feeling depressed or guilty
Before starting treatment, tell your doctor or pharmacist if you or your child have any of the above disorders or symptoms, as this medicine may worsen these problems. Your doctor will want to monitor the effect of the medicine on you or your child.
During treatment, children and adolescents may unexpectedly experience prolonged erections. The erections can be painful and occur at any time. It is essential to contact your doctor immediately if the erection lasts more than 2 hours, especially if it is painful.
Checks that your doctor will make before you or your child start treatment with methylphenidate
These checks are to decide if methylphenidate is the right medicine for you or your child. Your doctor will discuss these aspects with you:
- any other medicine that you or your child are taking
- if you or your child have a family history of sudden, unexplained death
- any other medical problem (such as heart problems) that you, your child, or your family may have
- how you or your child feel, for example, feeling elated or depressed, having unusual thoughts, or if you or your child have had these feelings in the past
- if there is a family history of "tics" (difficulty controlling oneself, repeated contractions of any part of the body, or repetition of sounds and words)
- any mental health or behavioral problem that you or your child or other family members have or have had in the past. Your doctor will explain if you or your child are at risk of mood changes (going from mania to depression - called "bipolar disorder"). Your doctor will check your mental health history and family history of suicide, bipolar disorder, or depression
It is essential to provide as much information as possible. This information will help your doctor decide if methylphenidate is the right medicine for you or your child. Your doctor may think that you or your child need other medical tests before starting to take this medicine.
Drug tests
This medicine may give positive results in drug tests.
Effects in case of improper use as a doping agent
The use of methylphenidate may lead to positive results in doping tests.
Improper use of methylphenidate for doping purposes can put health at risk.
Other medicines and Rubifen Prolong
Tell your doctor or pharmacist if you or your child are taking, have recently taken, or might take any other medicines.
Do not take methylphenidate if you or your child:
- are taking a medicine called a "monoamine oxidase inhibitor (MAOI)" used to treat depression, or have taken an MAOI in the last 14 days. Taking an MAOI with methylphenidate can cause a sudden increase in blood pressure (see "Do not take Rubifen Prolong").
If you or your child are taking other medicines, methylphenidate may affect their action or cause side effects. Therefore, it may be necessary to change the dose of the medicine or stop treatment together. If you or your child are taking any of the following medicines, consult your doctor or pharmacist before taking methylphenidate:
- other medicines for depression
- medicines for severe mental health problems (such as schizophrenia)
- medicines for epilepsy
- medicines used to lower or increase blood pressure
- some cough and cold remedies whose ingredients may affect blood pressure. It is essential to consult your pharmacist when buying any of these medicines
- medicines that thin the blood to prevent clots
If you are unsure whether any of the medicines you or your child are taking are included in the above list, ask your doctor or pharmacist before taking methylphenidate.
Before an operation
Tell your doctor if you or your child are going to have an operation. You should not take methylphenidate on the day of the operation if a certain type of anesthesia is used, as there is a risk of a sudden increase in blood pressure during the procedure.
Taking Rubifen Prolong with food, drinks, and alcohol
Do not drink alcohol while taking this medicine, as alcohol may worsen its side effects. Remember that some foods and medicines contain alcohol.
Pregnancy, breastfeeding, and fertility
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor before using this medicine.
Available data do not suggest an increased risk of total congenital anomalies, although a small increased risk of cardiac malformations during its use in the first three months of pregnancy could not be ruled out. Your doctor will provide more information about this risk.
Consult your doctor or pharmacist before using methylphenidate if you or your daughter:
- are sexually active. Your doctor will discuss contraceptive methods with you
- are pregnant or think you may be pregnant. Your doctor will decide if you should take methylphenidate
- are breastfeeding or plan to breastfeed. It is possible that methylphenidate may pass into breast milk. Therefore, your doctor will decide if you should breastfeed while taking methylphenidate.
Driving and using machines
Dizziness, drowsiness, and visual disturbances may occur during treatment with methylphenidate. If these side effects occur, it may be dangerous to perform activities that require attention (such as driving, operating machinery, riding a bicycle, or climbing trees) until you are sure that you or your child are not affected.
Rubifen Prolong contains sucrose
Rubifen Prolong contains sucrose (a type of sugar). If your doctor has told you that you have an intolerance to certain sugars, consult them before taking this medicine.
3. How to Take Rubifen Prolong
Follow the administration instructions for this medication exactly as indicated by your doctor. If in doubt, consult your doctor or pharmacist again.
Dose
Usually, your doctor will start treatment with a low dose that will be gradually increased as needed.
Children (from 6 years old) and Adolescents
The recommended initial dose is 20 mg once a day. According to the doctor's judgment, treatment with methylphenidate can also be initiated with a dose of 10 mg. The maximum daily dose is 60 mg. Rubifen Prolong is taken once a day in the morning in patients under 18 years old.
Adults
- If it's the first time you're taking methylphenidate, the doctor will start treatment with a dose of 20 mg once a day and, if necessary, will gradually increase the dose in small weekly increments.
- If you have already received treatment with a modified-release methylphenidate formulation during childhood and have just turned 18, your doctor may maintain treatment at the same dose. If during childhood you were treated with an immediate-release formulation, your doctor will prescribe the equivalent dose of Rubifen Prolong.
The maximum daily dose is 80 mg.
For lower doses or smaller increments, different doses of this medication or other medications containing methylphenidate may be available.
Things Your Doctor Will Do When You or Your Child Are Under Treatment
Your Doctor Will Perform Some Tests:
- before you or your child start treatment: to ensure that methylphenidate is safe and beneficial (detailed in the section "Checks Your Doctor Will Perform Before You or Your Child Start Treatment with Methylphenidate").
- after you or your child have started treatment: these tests will be performed at least every 6 months, but possibly more frequently. These will also be performed when the dose is changed.
These tests will include:
- appetite check
- measurement of height and weight in children
- weight measurement in adults
- measurement of blood pressure and heart rate
- check for mood problems or other unusual sensations, or if these have worsened since taking Rubifen Prolong
Method of Administration
Rubifen Prolong is for oral use.
Take methylphenidate once a day in the morning. Methylphenidate should not be taken too late, as it can cause sleep disturbances.
- The capsule can be taken with or without food.
- The capsule must be swallowed whole, with a glass of water.
- Do not crush, chew, or divide the capsule or its contents.
If you or your child are unable to swallow the Rubifen Prolong capsule, you can pour the contents over a small amount of food, as follows:
- Open the capsule carefully and pour the granules over a small amount of soft food (e.g., apple sauce).
- The food should not be hot, as this could affect the special properties of the granules.
- Take the entire mixture of medication and food immediately.
Do not store any medication/food mixture for future use.
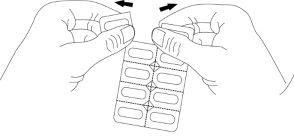
Instructions for Opening the Blister
This medication is available in child-resistant peelable blisters with a safety lock. Please follow the following instructions for opening the blister:
- Do not push the capsule to remove it from the blister, as this will cause it to crush.
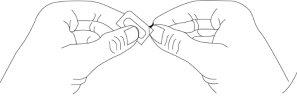
- Take the blister by the printed laminate facing upwards and fold it back at the perforated line, fold the blister in the opposite direction, and repeat several times. Separate at the perforated area to obtain a dose.
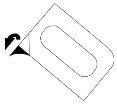
- To extract the capsule, carefully separate the laminate starting from the corner indicated with an arrow and pull it back.
Long-Term Treatment
Methylphenidate should not be taken for life. If the administration of methylphenidate lasts more than a year, your doctor should briefly suspend treatment at least once a year. In children, this can happen during school vacations. This will determine if the medication is still needed.
If You or Your Child Do Not Feel Better After 1 Month of Treatment
If you or your child do not feel better, inform your doctor. He may decide if you or your child need a different treatment.
Inadequate Use of Rubifen Prolong
If methylphenidate is not used properly, abnormal behavior may appear. It can also mean that you or your child start to depend on the medication. Inform your doctor if you or your child have abused or are dependent on alcohol, prescription medications, or drugs.
This medication is only for you or your child. Do not give this medication to other people, even if they have similar symptoms.
If You or Your Child Take More Rubifen Prolong Than You Should
If you or your child have taken more medication than you should, consult your doctor or call an ambulance immediately. Medical treatment may be necessary. You can also contact the Toxicology Information Service, phone 91 562 04 20.
Signs of overdose may include: vomiting, agitation, tremors, increased uncontrolled movements, muscle contractions, seizures (sometimes followed by coma), feeling extremely happy, confusion, seeing, feeling, or hearing things that are not real (hallucinations), sweating, flushing, headache, high fever, changes in heartbeats (slow, fast, or irregular), high blood pressure, dilated pupils, and dryness of the nose and mouth, muscle swelling, weak and sensitive muscles, muscle pain, dark urine.
If You or Your Child Forget to Take Rubifen Prolong
Do not take a double dose to make up for forgotten doses. If you or your child forget to take a dose, you should wait for the next dose at the scheduled time.
If You or Your Child Stop Treatment with Rubifen Prolong
If you or your child suddenly stop taking the medication, it is possible that ADHD symptoms or unwanted effects such as depression may reappear. Your doctor will recommend that you gradually reduce the amount of medication you take per day before stopping it completely. Consult your doctor before stopping methylphenidate.
If you have any other questions about the use of this medication, ask your doctor or pharmacist.
4. Possible Side Effects
Like all medications, this medication can cause side effects, although not everyone will experience them. Your doctor will discuss these side effects with you.
Some Side Effects Could Be Serious. If You or Your Child Experience Any of the Following Side Effects, Inform Your Doctor Immediately:
Frequent (May Affect Up to 1 in 10 People)
- irregular heartbeats (palpitations)
- changes in personality
- excessive teeth grinding (bruxism)
Uncommon (May Affect Up to 1 in 100 People)
- suicidal thoughts or feelings
- seeing, feeling, or hearing things that are not real, symptoms of psychosis
- uncontrolled body movements or speech (Tourette's syndrome)
- allergic reaction signs such as rash, itching, or hives on the skin, swelling of the face, lips, tongue, or other parts of the body, shortness of breath, difficulty breathing
- mood changes or disturbances
Rare (May Affect Up to 1 in 1,000 People)
- feeling exceptionally excited, more active than usual, or uninhibited (mania)
Very Rare (May Affect Up to 1 in 10,000 People)
- heart attack
- seizures (epilepsy with convulsions)
- skin peeling or red-purple spots
- uncontrolled muscle spasms affecting the eyes, head, neck, body, and nervous system due to lack of blood circulation to the brain
- paralysis or movement problems and vision problems, speech difficulties; can be signs of problems in the blood vessels of the brain
- decrease in the number of blood cells (red blood cells, white blood cells, and platelets) that can make you more prone to infections and cause easier bleeding and bruising
- sudden increase in body temperature, very high blood pressure, and severe seizures ("neuroleptic malignant syndrome"). It is not entirely certain that this side effect is caused by methylphenidate or other medications taken in combination with methylphenidate
Unknown Frequency (Cannot Be Estimated from Available Data)
- unwanted recurring thoughts
- unexplained fainting, chest pain, shortness of breath (can be signs of heart problems)
- inability to control urine elimination (incontinence)
- jaw muscle spasm that makes it difficult to open the mouth (trismus)
- stuttering
Other Side Effects, If They Become Serious, Inform Your Doctor or Pharmacist:
Very Common (May Affect More Than 1 in 10 People)
- decreased appetite
- headache
- feeling nervous
- difficulty sleeping
- nausea
- dry mouth
Common (May Affect Up to 1 in 10 People)
- joint pain
- fever
- abnormal hair loss or decreased hair thickness (thinner hair)
- excessive sleepiness or drowsiness
- loss of appetite
- weight loss in adults
- panic attacks
- decreased sexual desire
- toothache
- itching, rash, or red itchy patches (urticaria)
- excessive sweating
- cough, sore throat, or throat and nose irritation, difficulty breathing, or chest pain
- changes in blood pressure (mainly high blood pressure)
- rapid heartbeats (tachycardia)
- cold hands and feet
- shakes or tremors, feeling dizzy, uncontrolled movements, feeling restless
- being unusually active
- feeling aggressive, nervous, restless, anxious, depressed, stressed, irritable, and having abnormal behavior, sleep problems, and fatigue
- stomach pain, diarrhea, stomach discomfort, indigestion, thirst, and vomiting. These side effects usually occur at the start of treatment and may decrease when taking the medication with food.
Uncommon (May Affect Up to 1 in 100 People)
- constipation
- chest discomfort
- inflammation of the mucous membranes of the stomach and small intestine
- blood in the urine
- double vision or blurred vision
- muscle pain, muscle contractions, muscle stiffness
- increased liver test results (seen in blood tests)
- anger, restlessness, or tearfulness, excessive awareness of the environment, tension
Rare (May Affect Up to 1 in 1,000 People)
- changes in sexual desire
- feeling disoriented
- dilated pupils, vision problems
- breast swelling in men
- skin redness, increased redness of skin rashes
Very Rare (May Affect Up to 1 in 10,000 People)
- heart attack
- sudden death
- muscle cramps
- small red spots on the skin
- inflammation or blockage of the arteries in the brain
- abnormal liver function, including liver failure and coma
- changes in test results, including liver and blood tests
- suicide attempt (including completed suicide), abnormal thoughts, lack of feelings or emotion, doing things over and over, obsessing over something
- feeling numbness in the fingers and toes, tingling, and color change from white to blue, and then to red with cold (Raynaud's phenomenon)
Unknown Frequency (Frequency Cannot Be Estimated from Available Data)
- migraine
- very high fever
- slow, fast, or irregular heartbeats, palpitations
- major epileptic seizure ("grand mal" convulsions)
- believing things that are not true, confusion
- severe stomach pain with a feeling of discomfort and vomiting
- problems in the blood vessels of the brain (stroke, cerebral arteritis, or cerebral occlusion)
- erectile dysfunction
- prolonged, sometimes painful erections, or increased number of erections
- excessive and uncontrolled speech
- nosebleeds
Effects on Growth
When used for more than a year, methylphenidate may reduce growth rate in some children. This affects less than 1 in 10 children.
- There may be a lack of weight or height gain.
- Your doctor will closely monitor height, weight, and how you or your child are eating.
- If your child is not growing as expected, treatment with methylphenidate may be suspended for a short time.
Reporting Side Effects
If you experience any side effects, consult your doctor or pharmacist, even if they are possible side effects not listed in this leaflet. You can also report them directly through the Spanish Medication Monitoring System for Human Use: https://www.notificaram.es. By reporting side effects, you can contribute to providing more information on the safety of this medication.
5. Storage of Rubifen Prolong
Keep this medication out of sight and reach of children.
Do not use this medication after the expiration date shown on the box and blister after CAD. The expiration date is the last day of the month indicated.
This medication does not require special storage conditions.
Medications should not be disposed of in wastewater or household waste. Deposit the packaging and medications you no longer need at the pharmacy's SIGRE point. Ask your pharmacist how to dispose of the packaging and medications you no longer need. This will help protect the environment.
6. Container contents and additional information
Composition of Rubifen Prolong
- The active ingredient is methylphenidate hydrochloride.
Rubifen Prolong 10 mg modified-release hard capsules EFG contain 10 mg of methylphenidate hydrochloride (equivalent to 8.65 mg of methylphenidate).
Rubifen Prolong 20 mg modified-release hard capsules EFG contain 20 mg of methylphenidate hydrochloride (equivalent to 17.3 mg of methylphenidate).
Rubifen Prolong 30 mg modified-release hard capsules EFG contain 30 mg of methylphenidate hydrochloride (equivalent to 25.95 mg of methylphenidate).
Rubifen Prolong 40 mg modified-release hard capsules EFG contain 40 mg of methylphenidate hydrochloride (equivalent to 34.6 mg of methylphenidate).
Rubifen Prolong 60 mg modified-release hard capsules EFG contain 60 mg of methylphenidate hydrochloride (equivalent to 51.9 mg of methylphenidate).
- The other components are:
Capsule content: ammonio methacrylate copolymer (type B), methacrylic acid and methyl methacrylate copolymer (1:1), povidone 30, sugar spheres (containing sucrose and corn starch), talc, triethyl citrate.
Capsule shell: gelatin, titanium dioxide (E171).
In addition, in Rubifen Prolong 10 mg modified-release hard capsules, Rubifen Prolong 30 mg modified-release hard capsules, Rubifen Prolong 40 mg modified-release hard capsules, and Rubifen Prolong 60 mg modified-release hard capsules: Yellow iron oxide (E172).
Printing ink: potassium hydroxide, propylene glycol, red iron oxide (E172), shellac, concentrated ammonia solution.
Appearance of the product and container contents
Rubifen Prolong 10 mg modified-release hard capsules EFG
Opaque hard gelatin capsule (size 2) with a dark yellow cap and a white body, printed with "RUB" in red ink on the cap and "M10" in red ink on the body, containing white and off-white pellets. Capsule length: 18 mm.
Rubifen Prolong 20 mg modified-release hard capsules EFG
Opaque hard gelatin capsule (size 2) with a white cap and a white body, printed with "RUB" in red ink on the cap and "M20" in red ink on the body, containing white and off-white pellets. Capsule length: 18 mm.
Rubifen Prolong 30 mg modified-release hard capsules EFG
Opaque hard gelatin capsule (size 2) with an ivory cap and an ivory body, printed with "RUB" in red ink on the cap and "M30" in red ink on the body, containing white and off-white pellets. Capsule length: 18 mm.
Rubifen Prolong 40 mg modified-release hard capsules EFG
Opaque hard gelatin capsule (size 1) with a dark yellow cap and a dark yellow body, printed with "RUB" in red ink on the cap and "M40" in red ink on the body, containing white and off-white pellets. Capsule length: 20 mm.
Rubifen Prolong 60 mg modified-release hard capsules EFG
Opaque hard gelatin capsule (size 0) with a dark yellow cap and an ivory body, printed with "RUB" in red ink on the cap and "M60" in red ink on the body, containing white and off-white pellets. Capsule length: 22 mm.
Child-resistant safety blisters (Aclar/PVC//Al/PET) in boxes.
Formats:
10 mg: 28, 30, 50, 56, 60, 100 capsules.
20 mg: 28, 30, 50, 56, 60, 84, 100 capsules.
30 mg: 28, 30, 50, 54, 56, 60, 100 capsules.
40 mg: 28, 30, 50, 54, 56, 60, 100 capsules.
60 mg: 28, 30, 40, 50, 56, 60, 100 capsules.
Only some pack sizes may be marketed.
Marketing authorization holder and manufacturer
Laboratorios Rubió, S.A.
Industria, 29. Pol. Ind. Comte de Sert
08755 Castellbisbal (Barcelona)
Spain
This medicinal product is authorized in the Member States of the European Economic Area under the following names:
Germany Methylphenidat NewLine Pharma Hartkapseln mit veränderter Wirkstofffreisetzung
Spain Rubifen Prolong modified-release hard capsules EFG
Poland Symkinet MR
Portugal Metilfenidato NewLine Pharma
Date of last revision of this leaflet: March 2023
Detailed information on this medicinal product is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) (http://www.aemps.gob.es)
- Country of registration
- Average pharmacy price22.23 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to RUBIFEN PROLONG 40 mg PROLONGED-RELEASE HARD CAPSULESDosage form: MODIFIED-RELEASE TABLET, 18 mgActive substance: methylphenidateManufacturer: Exeltis Healthcare S.L.Prescription requiredDosage form: MODIFIED-RELEASE TABLET, 27 mgActive substance: methylphenidateManufacturer: Exeltis Healthcare S.L.Prescription requiredDosage form: MODIFIED-RELEASE TABLET, 36 mgActive substance: methylphenidateManufacturer: Exeltis Healthcare S.L.Prescription required
Online doctors for RUBIFEN PROLONG 40 mg PROLONGED-RELEASE HARD CAPSULES
Discuss questions about RUBIFEN PROLONG 40 mg PROLONGED-RELEASE HARD CAPSULES, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions













