ROCURONIUM AGUETTANT 10 mg/mL Injectable Solution in Pre-filled Syringe

How to use ROCURONIUM AGUETTANT 10 mg/mL Injectable Solution in Pre-filled Syringe
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet:information for the user
Rocuronium Aguettant10mg/ml solution for injection in a pre-filled syringe
Rocuronium bromide
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- If you experience any side effects, talk to your doctor or pharmacist, even if it is not listed in this leaflet. See section 4.
Contents of the pack
- What is Rocuronium Aguettant and what is it used for
- What you need to know before you use Rocuronium Aguettant
- How to use Rocuronium Aguettant
- Possible side effects
5 Conservation of Rocuronium Aguettant
- Contents of the pack and further information
1. What is Rocuronium Aguettant and what is it used for
Rocuronium is a muscle relaxantused in adults and children from 2 years of age. Muscle relaxants are used during surgical procedures as adjuncts during general anesthesia. During a surgical procedure, your muscles must be completely relaxed. This makes it easier for the surgeon to perform the surgical procedure. Normally, nerves send messages to your muscles. Rocuronium works by temporarily blocking these signals, causing muscle relaxation. Since the muscles needed for breathing also relax, you will be given artificial respiration until you can breathe on your own again. During the surgical procedure, the effect of the muscle relaxant will be constantly monitored, and if necessary, more medication will be administered. At the end of the operation, the effects of rocuronium will be allowed to disappear, and you will be able to start breathing on your own. Sometimes, another medication will be given to speed up this process. Rocuronium can also be used in the Intensive Care Unit.
2. What you need to know before you use Rocuronium Aguettant
Do not use Rocuronium Aguettant
- if you are allergic to rocuronium or any of the other ingredients of this medicine (listed in section 6).
Consult your doctor if this applies to you.
Warnings and precautions
Your medical history may affect how Rocuronium Aguettant is administered to you. Inform your doctor if you have or have had any of the following diseases:
- allergy to muscle relaxants
- renal impairment (renal failure) or kidney disease
- cardiovascular disease
- edema formation (fluid retention, e.g., in the ankles)
- liver disease, gallbladder disease, or bile duct disease, or impaired liver function.
- diseases that affect the nerves and muscles.
- history of malignant hyperthermia (sudden fever with rapid heart rate, rapid breathing, and muscle stiffness, pain, and/or weakness).
Certain medical conditions may affect how Rocuronium Aguettant works. For example:
- low potassium levels in the blood (hypokalemia)
- high magnesium levels in the blood (hypermagnesemia), e.g., when treating pregnancy toxemia with magnesium salts
- low calcium levels in the blood (hypocalcemia)
- low protein levels in the blood (hypoproteinemia)
- fluid deficiency (dehydration)
- excess acid in the blood (acidosis)
- excess carbon dioxide in the blood (hypercapnia)
- general poor health
- overweight
- burns
If any of these conditions apply to you, your doctor will take them into account when deciding on the appropriate dose of Rocuronium Aguettant for you.
Children and adolescentsRocuronium Aguettant can be used in children (2-11 years) and adolescents (12-17 years). However, the maintenance dose is not indicated in the pediatric population under 12 years.
Rocuronium Aguettant should not be administered to children under 2 years because the sub-graduation of the pre-filled syringe does not allow for precise administration of the product in these populations.
Other medicines and Rocuronium Aguettant
Tell your doctor if you are using, have recently used, or might use any other medicines. This will help your doctor determine the correct dose of Rocuronium Aguettant for you.
The following medicines may affect the effect of Rocuronium Aguettant:
Medicines that increase the effect of Rocuronium Aguettant:
- certain anesthetics
- medicines used as muscle relaxants (suxamethonium)
- certain medicines used to treat bacterial infections (antibiotics)
- certain medicines used to treat manic-depressive illness (lithium)
- certain medicines for heart diseases or high blood pressure (quinidine, calcium antagonists, beta-blockers)
- certain medicines used to treat malaria (quinine)
- medicines that cause increased urine production (diuretics)
- magnesium salts
- local anesthetics (lidocaine and bupivacaine)
- short-term use of medicines for the treatment of epilepsy (phenytoin), such as during an operation.
Medicines that decrease the effect of Rocuronium Aguettant:
- prolonged use of corticosteroids (anti-inflammatory) or medicines for epilepsy (phenytoin and carbamazepine)
- medicines for pancreatitis, blood coagulation problems, and acute blood loss (protease inhibitors: gabexate, ulinastatin)
- Calcium chloride, potassium chloride.
Medicines with a variable effect on Rocuronium Aguettant:
- other medicines used to relax muscles.
Rocuronium Aguettant may affect the effects of the following medicines:
- May increase the effect of local anesthetics (such as lidocaine).
Pregnancy, breast-feeding and fertility
If you are pregnant or breast-feeding, think you may be pregnant, or are planning to have a baby, ask your doctor for advice before using this medicine.
There are limited data on the use of rocuronium bromide during human pregnancy, and no data on women during breast-feeding. Rocuronium Aguettant should only be administered to pregnant and breast-feeding women when the doctor decides that the benefits outweigh the risks.
This medicine can be administered during a cesarean section.
Driving and using machines
Your doctor will inform you when you can drive or use hazardous machines after the administration of Rocuronium Aguettant.
Rocuronium Aguettant contains sodium
This medicine contains less than 1 mmol of sodium (23 mg) per pre-filled syringe; it is essentially "sodium-free"
3. How to use Rocuronium Aguettant
Dosage
Your doctor will determine the dose of Rocuronium Aguettant based on:
- the type of anesthesia used
- the expected duration of the surgical procedure
- other medicines you are using
- your age and health status.
A healthcare professional will administer Rocuronium Aguettant to you before and/or during the surgical procedure. The usual dose is 0.6 mg of rocuronium bromide per kilogram of body weight, and the effect lasts for 30 to 40 minutes. During the procedure, it will be checked if rocuronium is still working. If necessary, additional doses will be administered.
How Rocuronium Aguettant is administered
Rocuronium is not intended for self-administration. Rocuronium will be injected into a vein. It will be administered as a single injection or through an infusion.
If you use more Rocuronium Aguettant than you should
Since the medical staff will carefully monitor your condition, it is unlikely that you will be given too much rocuronium. However, if this happens, artificial respiration will be maintained until you are able to breathe on your own again. It is possible to counteract the effects of (excess) rocuronium and speed up your recovery by administering a medicine that counteracts the effects of rocuronium.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them. If these side effects occur during anesthesia, they will be observed and treated by your doctor.
The following side effects may occur:
Rare or uncommon (may affect less than 1 in 100/1,000 people)
- increased heart rate (tachycardia)
- decreased blood pressure (hypotension)
- absence, increase, or decrease of the effect of Rocuronium Aguettant
- pain at the injection site
- redness or itching at the injection site
- prolongation of the muscle relaxant effect of Rocuronium Aguettant
- delayed recovery from anesthesia
Very rare (may affect less than 1 in 10,000 people)
- allergic reactions such as difficulty breathing, changes in blood pressure or heart rate, shock (abrupt drop in blood pressure) due to insufficient blood circulation, or skin changes (e.g., fluid accumulation, redness, or rash)
- excessive and prolonged contraction of the muscles of the airways, causing difficulty breathing (bronchospasm)
- muscle weakness or paralysis
- sudden accumulation of fluid in the skin and mucous membranes (e.g., throat or tongue), breathing difficulties, and/or itching or rash, often with an allergic reaction (angioedema)
- fluid accumulation (edema) in the face
- breathing difficulties due to anesthesia
- skin rash, sometimes with intense itching and hives (urticaria)
- redness of the skin
- flushing
Unknown frequency (cannot be estimated from the available data)
- allergic spasm and severe coronary artery vasospasm (Kounis syndrome) causing chest pain (angina) or heart attack (myocardial infarction).
- dilated pupils (mydriasis) or fixed pupils that do not change size with light or other stimuli
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist, even if it is not listed in this leaflet. You can also report side effects directly through the Spanish Medicines Agency's website: www.notificaRAM.es/. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Conservation of Rocuronium Aguettant
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the label, blister, and carton.
Store in a refrigerator (2°C - 8°C).
Do not freeze.
Store the pre-filled syringe in its blister pack until use.
Once opened, the medicine must be used immediately.
This medicine can be stored at a temperature up to 30°C for a maximum of 12 weeks. In all cases, once removed from the refrigerator, the medicine must be discarded after 12 weeks.
The product must not be re-introduced into the refrigerator once stored outside. The shelf life cannot exceed the expiry date stated on the packaging.
Do not use this medicine if you notice visible signs of deterioration.
Any pre-filled syringe, even if partially used, must be disposed of properly after use.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Contents of the pack and further information
Composition of Rocuronium Aguettant
- The active substance is rocuronium bromide.
Each ml of solution contains 10 mg of rocuronium bromide.
Each 5 ml pre-filled syringe contains 50 mg of rocuronium bromide (50 mg/5 ml).
- The other ingredients are sodium acetate trihydrate (E 262), sodium chloride, glacial acetic acid (E260), and water for injections.
Appearance of the product and pack contents
Rocuronium Aguettant is a solution for injection or infusion, from colorless to pale yellowish-brown, in a 5 ml pre-filled syringe, with a transparent graduated label (sub-graduations of 0.2 ml, from 0 to 5 ml). Each pre-filled syringe is packaged individually in a transparent blister pack.
Pack size: cardboard boxes of 10 pre-filled syringes.
Marketing authorization holder and manufacturer
Marketing authorization holder
Laboratoire Aguettant
1 rue Alexander Fleming,
69007 Lyon
France
Manufacturer
Laboratoire Aguettant
1, rue Alexander Fleming
69007 LYON
France
Laboratoire Aguettant
Lieu Dit Chantecaille
07340 Champagne
France
This medicine is authorized in the Member States of the European Economic Area under the following names:
AT: Rocuroniumbromid Aguettant
FR, DE, IS, PL, RO: Rocuronium Aguettant
BE, DK, FI, LU, NO, SE: Rocuronium bromide Aguettant
IT: Rocuronio bromuro Aguettant
NL: Rocuroniumbromide Aguettant
PT: Brometo de Rocurónio Aguettant
ES: Rocuronio Aguettant 10mg/ml solution for injection in a pre-filled syringe
IE: Rocuronium bromide
Date of last revision of this leaflet: May 2025.
Detailed information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es
----------------------------------------------------------------------------------------------------------------------------------
This information is intended only for healthcare professionals:
The pre-filled syringe is not suitable for the precise administration of the product in children under 2 years.
The pre-filled syringe is for single patient use only. Discard the syringe after use. Do not reuse.
Appearance of the solution
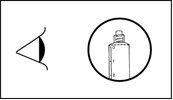
The product should be visually inspected for particles and color changes before administration. Only a clear solution from colorless to pale yellowish-brown without particles or precipitates should be used.
Incompatibilities of the solution
Rocuronium bromide is physically incompatible with solutions of the following medicines: amphotericin, amoxicillin, azathioprine, cefazolin, cloxacillin, dexamethasone, diazepam, enoximone, erythromycin, famotidine, furosemide, sodium hydrocortisone succinate, insulin, methohexital, methylprednisolone, prednisolone sodium succinate, thiopental, trimethoprim, and vancomycin.
Rocuronium bromide is also incompatible with intralipid.
Use of the pre-filled syringe
The content of an unopened and undamaged blister is sterile, and the blister should not be opened until the syringe is ready for use.
The pre-filled syringe is for single patient use only. Discard the syringe after use. Do not reuse.
The pre-filled syringe is not suitable for the precise administration of the product in children under 2 years.
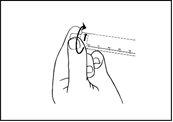
The product should not be used if the security seal of the syringe is broken.
Do not use this medicine if you notice visible signs of deterioration.
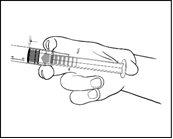
Please prepare the syringe carefully as follows
The outer surface of the syringe is sterile until the blister is opened. The blister should not be opened until use.
If handled with an aseptic method, this medicine can be placed in a sterile field once removed from the blister.
- Remove the pre-filled syringe from the blister.
|
|
|
|
|
|
|
|
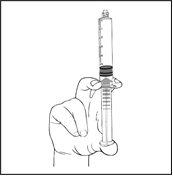
- Connect the syringe to the vascular access device using a luer/luer lock system. Slowly push the plunger to inject the required volume. Administer the product through the appropriate administration route.
The pre-filled syringe is not suitable for syringe drivers. The pre-filled syringe is a ready-to-administer product and is not suitable for dilution in an infusion bag.
No syringe that has been damaged or manipulated without respecting the conditions of sterility should be used.
Any unused product or waste material should be disposed of in accordance with local regulations.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to ROCURONIUM AGUETTANT 10 mg/mL Injectable Solution in Pre-filled SyringeDosage form: INJECTABLE, 10 mg rocuronium bromide/ mlActive substance: rocuronium bromideManufacturer: Schering Plough S.A.Prescription requiredDosage form: INJECTABLE, 10 mg/mlActive substance: rocuronium bromideManufacturer: B. Braun Melsungen AgPrescription requiredDosage form: INJECTABLE, 10 mg/mlActive substance: rocuronium bromideManufacturer: Hikma Farmaceutica (Portugal) S.A.Prescription required
Online doctors for ROCURONIUM AGUETTANT 10 mg/mL Injectable Solution in Pre-filled Syringe
Discuss questions about ROCURONIUM AGUETTANT 10 mg/mL Injectable Solution in Pre-filled Syringe, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions