RINVOQ 15 mg PROLONGED-RELEASE TABLETS


How to use RINVOQ 15 mg PROLONGED-RELEASE TABLETS
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
RINVOQ 15 mg prolonged-release tablets
RINVOQ 30mg prolonged-release tablets
RINVOQ 45mg prolonged-release tablets
upadacitinib
Read this entire leaflet carefully before you start taking this medicine, because it contains important information for you.
- Keep this leaflet, as you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you experience any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is RINVOQ and what is it used for
- What you need to know before you take RINVOQ
- How to take RINVOQ
- Possible side effects
- Storing RINVOQ
- Contents of the pack and other information
1. What is RINVOQ and what is it used for
RINVOQ contains the active substance upadacitinib. It belongs to a group of medicines called Janus kinase inhibitors. By reducing the activity of an enzyme in the body called "Janus kinase", RINVOQ reduces inflammation in the following diseases:
- Rheumatoid arthritis
- Psoriatic arthritis
- Axial spondyloarthritis,
- Non-radiographic axial spondyloarthritis
- Ankylosing spondylitis (AS, radiographic axial spondyloarthritis)
- Giant cell arteritis
- Atopic dermatitis
- Ulcerative colitis
- Crohn's disease
Rheumatoid arthritis
RINVOQ is used to treat adults with rheumatoid arthritis. Rheumatoid arthritis is a disease that causes inflammation of the joints. If you have moderate to severe rheumatoid arthritis, you may first be prescribed other medicines, one of which is usually methotrexate. If these medicines do not work well enough, you will be given RINVOQ alone or in combination with methotrexate to treat your rheumatoid arthritis.
RINVOQ may help reduce pain, stiffness, and inflammation in your joints, reduce fatigue, and slow down the progression of damage to the bone and cartilage in your joints. These effects may make it easier for you to perform your daily activities and improve your quality of life.
Psoriatic arthritis
RINVOQ is used to treat adults with psoriatic arthritis. Psoriatic arthritis is a disease that causes inflammation of the joints and psoriasis. If you have active psoriatic arthritis, you will probably be given other medicines first. If these medicines do not work well enough, you will be given RINVOQ alone or in combination with methotrexate to treat your psoriatic arthritis.
RINVOQ may help reduce pain, stiffness, and inflammation in and around your joints, pain and stiffness in your spine, skin rash due to psoriasis, and fatigue, and may also slow down damage to the bones and cartilage in your joints. These effects may make it easier for you to perform your daily activities and improve your quality of life.
Axial spondyloarthritis (non-radiographic axial spondyloarthritis and ankylosing spondylitis)
RINVOQ is used to treat adults with axial spondyloarthritis. Axial spondyloarthritis is a disease that mainly causes inflammation in the spine. If you have active axial spondyloarthritis, you will probably be given other medicines first. If these medicines do not work well enough, you will be given RINVOQ to treat your axial spondyloarthritis.
RINVOQ may help reduce lower back pain, stiffness, and inflammation in your spine. These effects may make it easier for you to perform your daily activities and improve your quality of life.
Giant cell arteritis
RINVOQ is used to treat adults with giant cell arteritis. Giant cell arteritis is a disease that causes inflammation of the blood vessels, which usually affects the medium and large arteries in the head, neck, and arms.
RINVOQ may help control the signs and symptoms of giant cell arteritis, including headache, scalp tenderness, jaw pain, and fatigue. These effects may make it easier for you to perform your daily activities and improve your quality of life. Giant cell arteritis is usually treated with medicines called steroids. They are usually effective, but may have side effects if given in high doses or used for a long time. Reducing the dose of steroids may also lead to a flare-up of giant cell arteritis. Adding RINVOQ to treatment means that steroids can be used for a shorter time and, in turn, control giant cell arteritis.
Atopic dermatitis
RINVOQ is used to treat adults and adolescents from 12 years of age with moderate to severe atopic dermatitis, also known as atopic eczema. RINVOQ can be used with medicines for eczema that are applied to the skin or can be used alone.
Taking RINVOQ may improve the condition of your skin and reduce itching and flare-ups. RINVOQ may help improve symptoms of pain, anxiety, and depression that people with atopic dermatitis may have. RINVOQ may help improve sleep disorders and overall quality of life.
Ulcerative colitis
Ulcerative colitis is an inflammatory disease of the large intestine. RINVOQ is used to treat adults with ulcerative colitis who have not responded sufficiently or did not tolerate previous treatment.
RINVOQ may help reduce the signs and symptoms of the disease, including bloody stools, abdominal pain, and the urge to go to the bathroom and frequency of going to the bathroom. These effects may make it easier for you to perform your daily activities and reduce fatigue.
Crohn's disease
Crohn's disease is an inflammatory disease that can affect any part of the digestive tract, but most often affects the intestine. RINVOQ is used to treat adults with Crohn's disease who have not responded sufficiently or did not tolerate previous treatment.
RINVOQ may help reduce the signs and symptoms of the disease, including the urge to go to the bathroom and frequency of going to the bathroom, abdominal pain, and inflammation of the lining of the intestine. These effects may make it easier for you to perform your daily activities and reduce fatigue.
2. What you need to know before you take RINVOQ
Do not take RINVOQ
- if you are allergic to upadacitinib or any of the other ingredients of this medicine (listed in section 6)
- if you have a severe infection (such as pneumonia or skin infection caused by bacteria)
- if you have active tuberculosis (TB)
- if you have severe liver problems
- if you are pregnant (see the section Pregnancy, breastfeeding, and contraception).
Warnings and precautions
Talk to your doctor or pharmacist before and during treatment with RINVOQ if:
- you have an infection or if you get infections often. Tell your doctor if you have symptoms such as fever, wounds, feeling more tired than usual, or dental problems, as these could be signs of infection. RINVOQ may reduce your body's ability to fight infections and may worsen an existing infection or increase the likelihood of getting a new infection. If you have diabetes or are 65 years of age or older, you may be more likely to get infections
- you have had tuberculosis or have been in close contact with someone with tuberculosis. Your doctor will do a test for tuberculosis before you start taking RINVOQ and may repeat the test during treatment
- you have had a herpes infection (herpes zoster), as RINVOQ may cause it to come back. Tell your doctor if you have a painful rash with blisters, as these may be signs of herpes zoster
- you have had hepatitis B or C
- you have been vaccinated recently or are scheduled to be vaccinated (immunization) – this is because live vaccines are not recommended while taking RINVOQ
- you have or have had cancer in the past, smoke or have smoked in the past, because your doctor will assess with you whether RINVOQ is suitable for you
- non-melanoma skin cancer has been observed in patients taking RINVOQ. Your doctor may recommend that you undergo regular skin examinations while taking RINVOQ. Tell your doctor if you notice any new skin lesions during or after treatment or if the appearance of existing lesions changes
- you have or have had heart problems, because your doctor will assess with you whether RINVOQ is suitable for you
- your liver is not working as well as it should
- you have had blood clots in the veins of your legs (deep vein thrombosis) or lungs (pulmonary embolism) or are at a higher risk of developing them (for example: if you have had recent major surgery, use hormonal contraceptives/hormone replacement therapy, if you or your close relatives have been identified with any coagulation disorder). Your doctor will assess with you whether RINVOQ is suitable for you. Tell your doctor if you experience sudden shortness of breath or difficulty breathing, chest pain or pain in the upper back, swelling in the leg or arm, pain or tenderness to the touch in the leg or arm, or redness or change in color in the leg or arm, as these may be signs of blood clots in the veins
- you experience sudden vision changes. Consult a doctor immediately if you experience sudden symptoms such as blurred vision or partial or total loss of vision, as these may be a sign of obstruction of blood flow in the eyes
- you have kidney problems
- you have stomach pain (abdominal) of unknown origin, have or have had diverticulitis (painful inflammation of small pouches in the lining of the intestine) or stomach or intestinal ulcers, or are taking non-steroidal anti-inflammatory drugs
- you repeatedly notice a tablet or parts of a tablet in your stool.
If you notice any of the following serious side effects, tell your doctor immediately:
- symptoms such as a rash (hives), difficulty breathing, or swelling of your lips, tongue, or throat, you may be having an allergic reaction. Some people taking RINVOQ have had serious allergic reactions. If you experience any of these symptoms while taking RINVOQ, stop taking RINVOQ and seek immediate emergency medical attention
- severe stomach pain, especially accompanied by fever, nausea, and vomiting.
Blood tests
You will need to have a blood test before you start taking RINVOQ, or while you are taking it. This is done to check if you have a low count of red blood cells (anemia), a low count of white blood cells (neutropenia or lymphopenia), high levels of fat in the blood (cholesterol), or high levels of liver enzymes. The tests are done to check that treatment with RINVOQ is not causing problems.
Elderly patients
There is a higher rate of infections in patients 65 years of age and older. Tell your doctor immediately if you notice any signs or symptoms of infection.
Patients 65 years of age and older may have a higher risk of infections, heart problems, including heart attack, and some types of cancer. Your doctor will assess with you whether RINVOQ is suitable for you.
Children and adolescents
RINVOQ is not recommended for use in children with atopic dermatitis under 12 years of age or adolescents with a weight below 30 kg. It has not been studied in these patients.
RINVOQ is not recommended for use in children and adolescents under 18 years of age with rheumatoid arthritis, psoriatic arthritis, axial spondyloarthritis (non-radiographic axial spondyloarthritis and ankylosing spondylitis), ulcerative colitis, or Crohn's disease. It has not been studied in this age group.
Other medicines and RINVOQ
Tell your doctor or pharmacist if you are taking, have recently taken, or might take any other medicines. Some medicines may reduce the effect of RINVOQ or increase the risk of side effects. It is very important that you tell your doctor or pharmacist if you are taking any of the following:
- medicines to treat fungal infections (such as itraconazole, posaconazole, or voriconazole)
- medicines to treat bacterial infections (such as clarithromycin)
- medicines to treat Cushing's syndrome (such as ketoconazole)
- medicines to treat tuberculosis (such as rifampicin)
- medicines to treat seizures or epilepsy (such as phenytoin)
- medicines that affect your immune system (such as azathioprine, 6-mercaptopurine, cyclosporine, and tacrolimus)
- medicines that may increase the risk of gastrointestinal perforation or diverticulitis, such as non-steroidal anti-inflammatory drugs (usually used to treat painful and/or inflammatory diseases of the muscles or joints), and/or opioids (used to treat severe pain), and/or corticosteroids (usually used to treat inflammatory diseases)
- medicines to treat diabetes or if you have diabetes. Your doctor may decide if you need less diabetes medicine while taking upadacitinib.
If you experience any of these or are unsure, consult your doctor or pharmacist before taking RINVOQ.
Pregnancy, breastfeeding, and fertility
Pregnancy
RINVOQ must not be used during pregnancy.
Breastfeeding
If you are breastfeeding or plan to breastfeed, consult your doctor before taking this medicine. You must not use RINVOQ while breastfeeding, as it is not known whether this medicine passes into breast milk. You and your doctor must decide whether to breastfeed or take RINVOQ. You must not do both.
Fertility
If you are a woman of childbearing age, you must use an effective method of contraception to avoid becoming pregnant while taking RINVOQ and for at least 4 weeks after the last dose of RINVOQ. If you become pregnant during this time, you must tell your doctor immediately.
You must tell your doctor if your daughter has her first menstrual period while taking RINVOQ.
Driving and using machines
Do not drive or use machines if you feel dizzy or experience vertigo while taking RINVOQ, until these symptoms disappear.
3. How to Take RINVOQ
Follow your doctor's or pharmacist's instructions for taking this medication exactly.If you are unsure, consult your doctor or pharmacist again.
Dose to Take
If you have rheumatoid arthritis, psoriatic arthritis, axial spondyloarthritis (axial spondyloarthritis without radiographic evidence and ankylosing spondylitis), or giant cell arteritis
The recommended dose is one 15 mg tablet once a day.
If you have atopic dermatitis
Adults (18 to 64 years of age):
The recommended dose is 15 mg or 30 mg, as prescribed by your doctor, as one tablet once a day.
Your doctor may increase or decrease your dose depending on how you respond to treatment.
Adolescents (12 to 17 years of age) who weigh at least 30 kg:
The recommended dose is one 15 mg tablet once a day. Your doctor may increase your dose to one 30 mg tablet once a day depending on how you respond to treatment.
Elderly patients:
If you are 65 years of age or older, the recommended dose is 15 mg once a day.
If you have ulcerative colitis
The recommended dose is one 45 mg tablet once a day for 8 weeks. Your doctor will decide to extend the initial dose of 45 mg for an additional 8 weeks (a total of 16 weeks), followed by one 15 mg or 30 mg tablet once a day for long-term treatment. Your doctor may increase or decrease your dose depending on how you respond to the medication.
Elderly patients:
If you are 65 years of age or older, the recommended dose is 15 mg once a day for long-term treatment.
Your doctor may reduce your dose if you have kidney problems or if you are taking other medications.
If you have Crohn's disease
The recommended dose is one 45 mg tablet once a day for 12 weeks. This will be followed by one 15 mg or 30 mg tablet once a day for long-term treatment. Your doctor may increase or decrease your dose depending on how you respond to the medication.
Elderly patients:
If you are 65 years of age or older, the recommended dose is 15 mg once a day for long-term treatment.
Your doctor may reduce your dose if you have kidney problems or if you are taking other medications.
How to Take the Medication
- Swallow the tablet whole with water. Do not split, crush, chew, or break the tablet before swallowing, as this may alter the amount of medication that enters your body.
- To help you remember to take RINVOQ, take it at the same time every day.
- The tablets can be taken with or without food.
- Do not ingest the desiccant.
- Avoid foods or drinks that contain grapefruit while taking (or being treated with) RINVOQ, as they may make adverse effects more likely by increasing the amount of medication in your body.
If You Take More RINVOQ Than You Should
If you take more RINVOQ than you should, consult your doctor. You may experience some of the adverse effects described in section 4.
If You Miss a Dose of RINVOQ
- If you miss a dose, take it as soon as you remember.
- If you miss a dose for an entire day, simply skip the missed dose and take one dose the next day as usual.
- Do not take a double dose to make up for missed doses.
If You Stop Taking RINVOQ
Do not stop taking RINVOQ unless your doctor tells you to stop taking it.
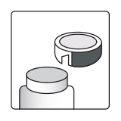
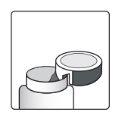
How to Open the Bottle
| Aluminum cutting device- on the bottle cap |
|
1a.Remove the bottle cap by pressing down and, without releasing, turn the cap counterclockwise. 1b.Turn the cap over and place the cutting device near the edge of the aluminum seal. |
|
|
|
|
If you have any further questions about using this medication, ask your doctor or pharmacist.
4. Possible Side Effects
Like all medications, this medication can cause side effects, although not everyone will experience them.
Serious Side Effects
Tell your doctor or seek medical help immediately if you experience any signs of:
- infection, such as shingles or painful skin rash with blisters (shingles) – frequent (may affect up to 1 in 10 people).
- lung infection (pneumonia), which can cause difficulty breathing, fever, and cough with mucus – frequent (may affect up to 1 in 10 people).
- blood infection (sepsis) – uncommon (may affect up to 1 in 100 people).
- allergic reaction (chest tightness, wheezing, swelling of the lips, tongue, or throat, hives) – uncommon (may affect up to 1 in 100 people).
Other Side Effects
Tell your doctor if you experience any of the following side effects:
Very Common(may affect more than 1 in 10 people)
- throat and nose infections
- acne
Common(may affect up to 1 in 10 people)
- non-melanoma skin cancer
- cough
- fever
- feverish sensations (simple herpes)
- feeling unwell in the stomach (nausea)
- increased levels of an enzyme called creatine kinase, observed in blood tests
- low white blood cell count in blood tests
- high cholesterol levels (a type of fat in the blood), observed in tests
- high liver enzyme levels, observed in blood tests (sign of liver problems)
- weight gain
- inflammation (swelling) of the hair follicles
- flu (influenza)
- anemia
- abdominal pain (abdomen)
- fatigue (unusual feeling of tiredness and weakness)
- headache (headache was very common in giant cell arteritis)
- hives (urticaria)
- urinary tract infection
- rash
- feeling of spinning (vertigo)
- dizziness
- lung infection (bronchitis)
- swelling of the feet and hands (peripheral edema)
Uncommon(may affect up to 1 in 100 people)
- oral candidiasis (white patches in the mouth)
- high triglyceride levels (a type of fat) in the blood, observed in tests
- diverticulitis (painful inflammation of small pouches in the intestine lining)
- gastrointestinal perforation (a hole in the intestine)
Additional Side Effects in Adolescents with Atopic Dermatitis
Common:
- warts (cutaneous papilloma)
Reporting Side Effects
If you experience any side effects, consult your doctor, pharmacist, or nurse, even if they are possible side effects not listed in this leaflet. You can also report them directly through the national reporting system included in Appendix V. By reporting side effects, you can help provide more information on the safety of this medication.
5. Storage of RINVOQ
Keep this medication out of sight and reach of children.
Do not use this medication after the expiration date shown on the blister pack and carton after "EXP".
This medication does not require any special storage temperature.
Store in the original blister pack or bottle with the cap tightly closed to protect it from moisture.
Medications should not be disposed of through wastewater or household waste. Ask your pharmacist how to dispose of the packaging and any unused medication. This will help protect the environment.
6. Package Contents and Additional Information
RINVOQ Composition
The active ingredient is upadacitinib.
RINVOQ 15 mg prolonged-release tablets
- Each prolonged-release tablet contains upadacitinib hemihydrate, equivalent to 15 mg of upadacitinib.
- Other ingredients are:
- Tablet core: microcrystalline cellulose, mannitol, tartaric acid, hypromellose, anhydrous colloidal silica, magnesium stearate.
- Coating: polyvinyl alcohol, macrogol, talc, titanium dioxide (E171), red iron oxide (E172), black iron oxide (E172).
RINVOQ 30 mg prolonged-release tablets
- Each prolonged-release tablet contains upadacitinib hemihydrate, equivalent to 30 mg of upadacitinib.
- Other ingredients are:
- Tablet core: microcrystalline cellulose, mannitol, tartaric acid, hypromellose, anhydrous colloidal silica, magnesium stearate.
- Coating: polyvinyl alcohol, macrogol, talc, titanium dioxide (E171), red iron oxide (E172).
RINVOQ 45 mg prolonged-release tablets
- Each prolonged-release tablet contains upadacitinib hemihydrate, equivalent to 45 mg of upadacitinib.
- Other ingredients are:
- Tablet core: microcrystalline cellulose, mannitol, tartaric acid, hypromellose, anhydrous colloidal silica, magnesium stearate.
- Coating: polyvinyl alcohol, macrogol, talc, titanium dioxide (E171), yellow iron oxide (E172), and red iron oxide (E172).
Product Appearance and Package Contents
RINVOQ 15 mg prolonged-release tablets
RINVOQ 15 mg prolonged-release tablets are purple, oblong, and biconvex, engraved with "a15" on one side.
The tablets are available in blister packs or bottles.
RINVOQ is available in packs of 28 or 98 prolonged-release tablets and in multipacks of 84 prolonged-release tablets with 3 packs, each containing 28 prolonged-release tablets.
Each calendar blister pack contains 7 tablets.
RINVOQ is available in bottles with a desiccant containing 30 prolonged-release tablets, each pack contains 1 bottle (30-tablet pack) or 3 bottles (90-tablet pack).
RINVOQ 30 mg prolonged-release tablets
RINVOQ 30 mg prolonged-release tablets are red, oblong, and biconvex, engraved with "a30" on one side.
The tablets are available in blister packs or bottles.
RINVOQ is available in packs of 28 or 98 prolonged-release tablets.
Each calendar blister pack contains 7 tablets.
RINVOQ is available in bottles with a desiccant containing 30 prolonged-release tablets, each pack contains 1 bottle (30-tablet pack) or 3 bottles (90-tablet pack).
RINVOQ 45 mg prolonged-release tablets
RINVOQ 45 mg prolonged-release tablets are yellow to yellow-mottled, oblong, and biconvex, engraved with "a45" on one side.
The tablets are available in blister packs or bottles.
RINVOQ is available in packs of 28 prolonged-release tablets.
Each calendar blister pack contains 7 tablets.
RINVOQ is available in bottles with a desiccant containing 28 prolonged-release tablets, each pack contains 1 bottle.
Not all pack sizes may be marketed.
Marketing Authorization Holder
AbbVie Deutschland GmbH & Co. KG
Knollstrasse
67061 Ludwigshafen
Germany
Manufacturer
AbbVie S.r.l.
S.R. 148 Pontina, km 52 SNC
04011 Campoverde di Aprilia (Latina)
Italy
AbbVie Logistics B.V.
Zuiderzeelaan 53
Zwolle, 8017 JV,
Netherlands
You can request more information about this medication by contacting the local representative of the marketing authorization holder:
Belgium/Belgique/Belgien AbbVie SA Tel: +32 10 477811 | Lithuania AbbVie UAB Tel: +370 5 205 3023 | |
| Luxembourg/Luxemburg AbbVie SA Belgique/Belgien Tel: +32 10 477811 | |
Czech Republic AbbVie s.r.o. Tel: +420 233 098 111 | Hungary AbbVie Kft. Tel.: +36 1 455 8600 | |
Denmark AbbVie A/S Tlf: +45 72 30-20-28 | Malta V.J.Salomone Pharma Limited Tel: +356 2122017 | |
Germany AbbVie Deutschland GmbH & Co. KG Tel: 00800 222843 33 (toll-free) Tel: +49 (0) 611 / 1720-0 | Netherlands AbbVie B.V. Tel: +31 (0)88 322 2843 | |
Estonia AbbVie OU Tel: +372 623 1011 | Norway AbbVie AS Tlf: +47 67 81 80 00 | |
Greece AbbVie ΦΑΡΜΑΚΕΥΤΙΚΗ Α.Ε. Τηλ: +30 214 4165 555 | Austria AbbVie GmbH Tel: +43 1 20589-0 | |
Spain AbbVie Spain, S.L.U. Tel.: +34 91 384 09 10 | Poland AbbVie Polska Sp. z o.o. Tel.: +48 22 372 78 00 | |
France AbbVie Tél: +33 (0) 1 45 60 13 00 | Portugal AbbVie, Lda. Tel: +351 (0)21 1908400 | |
Croatia AbbVie d.o.o. Tel + 385 (0)1 5625 501 | Romania AbbVie S.R.L. Tel: +40 21 529 30 35 | |
Ireland AbbVie Limited Tel: +353 (0)1 4287900 | Slovenia AbbVie Biofarmacevtska družba d.o.o. Tel: +386 (1)32 08 060 | |
Iceland Vistor Tel: +354 535 7000 | Slovakia AbbVie s.r.o. Tel: +421 2 5050 0777 | |
Italy AbbVie S.r.l. Tel: +39 06 928921 | Finland AbbVie Oy Puh/Tel: +358 (0)10 2411 200 | |
Cyprus Lifepharma (Z.A.M.) Ltd Τηλ.: +357 22 34 74 40 | Sweden AbbVie AB Tel: +46 (0)8 684 44 600 | |
Latvia AbbVie SIA Tel: +371 67605000 |
Date of Last Revision of this Leaflet:
Other Sources of Information
Detailed information about this medication is available on the European Medicines Agency website: http://www.ema.europa.eu.
You can also consult detailed and up-to-date information about this product by scanning the QR code below or on the outer packaging with a smartphone. The same information is also available at the following URL: www.rinvoq.eu.
Include QR code
To Listen to or Request a Copy of this Leaflet in
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to RINVOQ 15 mg PROLONGED-RELEASE TABLETSDosage form: MODIFIED-RELEASE TABLET, 30 mgActive substance: upadacitinibManufacturer: Abbvie Deutschland Gmbh & Co. KgPrescription requiredDosage form: MODIFIED-RELEASE TABLET, 45 MGActive substance: upadacitinibManufacturer: Abbvie Deutschland Gmbh & Co. KgPrescription requiredDosage form: TABLET, 100 mgActive substance: filgotinibManufacturer: Alfasigma S.P.A.Prescription required
Online doctors for RINVOQ 15 mg PROLONGED-RELEASE TABLETS
Discuss questions about RINVOQ 15 mg PROLONGED-RELEASE TABLETS, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions