
REUSIN 8 mg/ml CUTANEOUS SPRAY SOLUTION

How to use REUSIN 8 mg/ml CUTANEOUS SPRAY SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Prospectus: Information for the user
Reusin 8 mg/ml solution for cutaneous spray
indomethacin
Read the entire prospectus carefully before starting to take this medication, as it contains important information for you.
- Keep this prospectus, as you may need to read it again.
- If you have any doubts, consult your doctor or pharmacist.
- This medication has been prescribed to you only, and you should not give it to others, even if they have the same symptoms as you, as it may harm them.
- If you experience adverse effects, consult your doctor or pharmacist, even if they are not listed in this prospectus. See section 4.
Contents of the prospectus
- What Reusin is and what it is used for
- What you need to know before starting to use Reusin
- How to use Reusin
- Possible adverse effects
5 Conservation of Reusin
- Contents of the package and additional information
1. What Reusin is and what it is used for
Reusin belongs to the group of medications called non-steroidal anti-inflammatory drugs for topical use.
Indomethacin, the active ingredient of this medication, acts by reducing pain and inflammation.
Reusin is indicated in adults for the treatment of mild and occasional pain and inflammation in processes such as: osteoarthritis and acute and post-traumatic musculoskeletal disorders such as tendinitis, tenosynovitis, sprains, and lower back pain.
2. What you need to know before starting to use Reusin
Do not use Reusin
- if you are allergic to indomethacin or any of the other components of this medication (listed in section 6).
- if you have experienced an allergic reaction (hypersensitivity) after taking acetylsalicylic acid or other non-steroidal anti-inflammatory drugs (NSAIDs).
- if you have any other skin condition in the area to be treated.
- if the inflammation is caused by an infection.
- if you are pregnant or breastfeeding.
- .
Warnings and precautions
Consult your doctor or pharmacist before starting to use Reusin.
- if you use the medication on large areas for long periods, as it may cause the active ingredient to pass into the blood in significant amounts and produce more adverse effects.
- do not use an occlusive dressing.
- Reusin should not be ingested.
- Do not apply Reusin to the eyes, mucous membranes, or open wounds.
- aspiration of the solution may cause irritation of the respiratory tract.
- as it contains alcohol, the solution may be highly flammable.
Children
This medication should not be used in children and adolescents under 18 years of age due to the lack of data on its safety and efficacy.
Other medications and Reusin
Inform your doctor or pharmacist if you are using, have recently used, or may need to use any other medication.
Certain medications may interact with Reusin. In these cases, it may be necessary to change the dose or interrupt treatment with some of the other medications.
In particular, consult your doctor or pharmacist if you are using medications:
- that increase urine excretion (diuretics) as well as other medications for hypertension, as Reusin may decrease their efficacy;
- for the treatment of arterial hypertension and certain heart problems (angiotensin-converting enzyme inhibitors or angiotensin II antagonists), as their use with Reusin may affect kidney function.
It is not recommended to apply cosmetics or other skin products to the affected area without consulting your doctor first.
Pregnancy, breastfeeding, and fertility
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medication.
Pregnancy
In case of pregnancy, the use of indomethacin is not recommended, as there is not enough information on the use of indomethacin in pregnant women when applied to the skin. Therefore, its use during pregnancy is not recommended.
Breastfeeding
The use of this medication is not recommended during breastfeeding. It is unknown whether indomethacin applied to the skin passes into breast milk, but it is known that it passes into breast milk when administered orally (capsules, tablets...).
Driving and using machines
No effects on the ability to drive and use machines have been described after cutaneous administration of indomethacin.
3. How to use Reusin
Follow exactly the administration instructions of this medication indicated by your doctor or pharmacist. In case of doubt, consult your doctor or pharmacist again.
Reusin is intended exclusively for cutaneous use (to be applied to the skin).
The recommended dose is:
Adults
The usual dose is to apply 5 to 10 sprays, 3 to 4 times a day, unless your doctor has given you other instructions. This recommendation may be modified based on the extent of the area to be treated.
For the treatment of large areas, it is recommended not to exceed 25 ml per day, equivalent to 200 mg of indomethacin (about 150 sprays)
Recommendations for correct application:
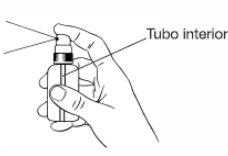
- When starting to use the package, press several times in a row to eliminate the air from the inner tube and achieve the correct exit of the liquid.
- Place the sprayer at a distance of 10 cm to 15 cm from the affected area and press the valve firmly the necessary number of times to complete the dosage.
- After application to the affected area, spread through a gentle massage to favor the penetration of the medication.
- It is recommended to wash your hands after using the medication.
Use in children
The use of Reusin is not recommended in children and adolescents under 18 years of age due to the lack of data on its safety and efficacy.
If you use more Reusin than you should
Since the application of this medication is for cutaneous use, it is unlikely that intoxication will occur. However, in case of accidental ingestion of large amounts, the symptoms of overdose may include nausea, disorientation, deafness, ringing in the ears, unusual excitement, convulsions, unusual drowsiness, rapid breathing, fever, and ultimately, unconsciousness and death.
In case of overdose or accidental ingestion, consult the Toxicology Information Service, phone 91 562 04 20, indicating the medication and the amount ingested. It is recommended to bring the package and the prospectus of the medication to the healthcare professional.
If you forget to use Reusin
Do not apply a double dose to make up for forgotten doses.
You should continue treatment as normal without taking any particular action.
If you stop treatment with Reusin
Stopping treatment with this medication does not require any particular action.
If you have any other doubts about the use of this medication, ask your doctor or pharmacist.
4. Possible adverse effects
Like all medications, this medication can cause adverse effects, although not all people experience them.
The adverse effects of indomethacin for cutaneous use are generally local, mild, and transient.
The possible adverse effects that may be observed with the use of this medication are listed below in decreasing order of frequency:
Rare adverse effects(may affect up to 1 in 1,000 patients):
- Itching, redness, and local eruptions.
- Local dryness and burning of the skin.
- Sun-induced skin inflammation (dermatitis).
Reporting adverse effects
If you experience any type of adverse effect, consult your doctor or pharmacist, even if it is a possible adverse effect that is not listed in this prospectus. You can also report them directly through the Spanish Medication Surveillance System for Human Use: https://www.notificaram.es. By reporting adverse effects, you can contribute to providing more information on the safety of this medication.
5. Conservation of Reusin
Keep this medication out of sight and reach of children.
This medication does not require special storage conditions.
Do not expose the package to fire. Reusin contains alcohol, making it a flammable product.
Do not use Reusin after the expiration date shown on the package, after CAD. The expiration date is the last day of the month indicated.
Medications should not be thrown away through the sewers or in the trash. Deposit the packages and medications you no longer need in the SIGRE point of the pharmacy. If in doubt, ask your pharmacist how to dispose of the packages and medications you no longer need. This way, you will help protect the environment.
6. Contents of the package and additional information
Composition of Reusin
- The active ingredient is indomethacin. Each ml of cutaneous spray solution contains 8 mg of indomethacin. Each spray contains 1.3 mg of indomethacin.
- The other components (excipients) are: isopropyl alcohol and isopropyl myristate.
Appearance of the product and contents of the package
Reusin 8 mg/ml Cutaneous Spray Solution is a transparent, yellow-green solution with a characteristic odor.
It is presented in a package containing 100 ml.
Marketing authorization holder
Laboratory STADA, S.L.
Frederic Mompou, 5
08960 Sant Just Desvern
(Barcelona)
Manufacturer
STADA Arzneimittel AG
Stadastrasse 2-18
61118 - Bad Vilbel
Germany
Date of the last revision of this prospectus: March 2021
Detailed and updated information on this medication is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to REUSIN 8 mg/ml CUTANEOUS SPRAY SOLUTIONDosage form: CREAM, 1.5 g aceclofenac / 100 gActive substance: aceclofenacManufacturer: Almirall S.A.Prescription requiredDosage form: GEL, 50 mg/gActive substance: ibuprofenManufacturer: Arafarma Group S.A.Prescription not requiredDosage form: GEL, 50 mg/gActive substance: ibuprofenManufacturer: Korhispana S.L.Prescription not required
Online doctors for REUSIN 8 mg/ml CUTANEOUS SPRAY SOLUTION
Discuss questions about REUSIN 8 mg/ml CUTANEOUS SPRAY SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions






