
RETSEVMO 80 mg HARD CAPSULES

How to use RETSEVMO 80 mg HARD CAPSULES
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Retsevmo 40mg hard capsules
Retsevmo 80mg hard capsules
selpercatinib
This medicine is subject to additional monitoring, which will allow for quick identification of new safety information. You can help by reporting any side effects you may get. The last section of the leaflet includes information on how to report side effects.
Read all of this leaflet carefully before you start taking this medicine, because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
- This leaflet was written as if the person taking the medicine is you. If you are giving this medicine to your child, replace “your” with “your child's” throughout this leaflet.
Contents of the pack
- What is Retsevmo and what is it used for
- What you need to know before you take Retsevmo
- How to take Retsevmo
- Possible side effects
- Storage of Retsevmo
- Contents of the pack and further information
1. What is Retsevmo and what is it used for
Retsevmo is a medicine for the treatment of cancer that contains the active substance selpercatinib.
It is used for the treatment of the following types of cancer that are caused by certain changes in the RET gene and that have spread and/or cannot be removed by surgery:
- A type of lung cancer called non-small cell lung cancer, in adults who have not been previously treated with a RET inhibitor medicine.
- Thyroid cancer (any type) in adults and adolescents aged 12 years and older if treatment with radioactive iodine, when appropriate, has not been able to control the cancer.
- A rare type of thyroid cancer called medullary thyroid cancer in adults and adolescents aged 12 years and older.
- Solid tumors (cancer) in other parts of the body in adults after previous treatments have not been able to control the cancer.
Your doctor will perform an analysis to check if your cancer has a change in the RET gene to ensure that Retsevmo is suitable for you.
How Retsevmo works
In patients with cancer that has a change in the RET gene, the change in the gene causes the body to produce an abnormal RET protein, which can cause uncontrolled cell growth and cancer. Retsevmo blocks the action of the abnormal RET protein and may therefore slow down or stop the growth of the cancer. It may also help reduce the size of the tumor.
If you have any questions about how Retsevmo works or why you have been prescribed it, ask your doctor.
2. What you need to know before you take Retsevmo
Do not take Retsevmo
- if you are allergic to selpercatinib or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Tell your doctor before you start taking Retsevmo:
- if you have lung or breathing problems other than lung cancer.
- if you have high blood pressure.
- if you have been told that you have a heart problem called prolonged QT interval after an electrocardiogram (ECG) test.
- if you have thyroid or thyroid hormone level problems.
- Retsevmo may affect fertility in women and men, which may affect your ability to have children. Talk to your doctor if this is a concern for you.
- if you have a recent history of significant bleeding.
Retsevmo may cause allergic reactions such as fever, rash, and pain. If you experience any of these reactions, talk to your doctor. After checking your symptoms, your doctor may ask you to take corticosteroids until your symptoms improve.
While taking Retsevmo, you may experience a rapid destruction of cancer cells (tumor lysis syndrome, TLS). This can cause irregular heartbeats, kidney failure, or abnormal blood test results. Talk to your doctor if you have a history of kidney problems or low blood pressure, as this may increase the risks associated with TLS.
Retsevmo may cause irregular growth or damage to the hip joint in pediatric patients (<18 years of age). if you experience hip or knee pain have a limp without known cause, talk to your doctor.< p>
See section 4, "Possible side effects", and talk to your doctor if you have any symptoms.
What your doctor will check before and during your treatment
- Retsevmo may cause severe, life-threatening, or fatal lung inflammation. Your doctor will monitor you before and during treatment with Retsevmo to detect any symptoms. Tell your doctor immediately if you notice any symptoms of lung problems, such as difficulty breathing, coughing, and increased temperature.
- Retsevmo may affect your blood pressure. Your blood pressure will be checked before and during treatment with Retsevmo.
- Retsevmo may affect how your liver works. Tell your doctor immediately if you have symptoms of liver problems, including yellowing of the skin and eyes (jaundice), loss of appetite, nausea or vomiting, or pain in the upper right part of your abdomen.
- Retsevmo may cause abnormal ECG results. An ECG will be performed before and during treatment with Retsevmo. Tell your doctor if you experience fainting, as this may be a symptom of an abnormal ECG.
- Retsevmo may affect your thyroid function. Your doctor will check your thyroid function before and during treatment with Retsevmo.
- You will have regular blood tests before and during treatment with Retsevmo to check your liver function and electrolyte levels (such as sodium, potassium, magnesium, and calcium) in your blood.
- If you are under 18 years of age, your doctor may monitor your growth during treatment. If you have hip, knee, or other leg pain, tell your doctor.
Children and adolescents
Retsevmo is not indicated for use in patients under 18 years of age with lung cancer.
The indications for thyroid cancer (including medullary thyroid cancer) do not cover children under 12 years of age.
Other medicines and Retsevmo
Tell your doctor or pharmacist if you are taking, have recently taken, or might take any other medicines.
Tell your doctor or pharmacist before taking Retsevmo, in particular if you are taking the following medicines:
- medicines that may increase the levels of Retsevmo in your blood:
- clarithromycin (used to treat bacterial infections)
- itraconazole, ketoconazole, posaconazole, voriconazole (used to treat fungal infections)
- atazanavir, ritonavir, cobicistat (used to treat HIV/AIDS)
- medicines that may reduce the effectiveness of Retsevmo:
- carbamazepine (used to treat epilepsy, nerve pain, bipolar disorder)
- rifampicin (used to treat tuberculosis (TB) and some other infections)
- St. John's Wort (a herbal remedy used to treat mild depression and anxiety)
- omeprazole, lansoprazole, or other proton pump inhibitors used to treat stomach acid, ulcers, and gastroesophageal reflux. If you are taking any of these medicines, take Retsevmo with a full meal.
- ranitidine, famotidine, and other H2 blockers used to treat ulcers and gastroesophageal reflux. If you are taking any of these medicines, take them 2 hours after taking Retsevmo.
- medicines whose levels in the blood may increase with Retsevmo:
- repaglinide (used to treat type 2 diabetes and control blood sugar)
- dasabuvir (used to treat hepatitis C)
- selexipag (used to treat pulmonary arterial hypertension)
- digoxin (used to treat heart conditions)
- lovastatin and simvastatin (used to treat high cholesterol)
- dabigatran (used to prevent and treat blood clots)
- medicines that may be less effective if taken with Retsevmo:
- levothyroxine (used to treat hypothyroidism)
Pregnancy, breastfeeding, and fertility
Pregnancy
If you are pregnant, think you may be pregnant, or are planning to have a baby, ask your doctor for advice before taking this medicine.
Do not take Retsevmo while you are pregnant, as its effects on the fetus are unknown.
Breastfeeding
Do not breastfeed while taking Retsevmo, as it may harm your baby. It is not known if Retsevmo passes into breast milk. Do not breastfeed for at least one week after your last dose of Retsevmo.
Contraception
Women are advised to avoid becoming pregnant and men are advised to avoid fathering a child during treatment with Retsevmo, as this medicine may harm the baby. If there is a possibility that the person taking this medicine may become pregnant or father a child, they should use adequate contraception during treatment and for at least one week after the last dose of Retsevmo.
Fertility
Retsevmo may affect your ability to have children. Talk to your doctor for advice on preserving fertility before starting treatment.
Driving and using machines
You should be careful when driving or using machines, as you may feel tired or dizzy while taking Retsevmo.
3. How to take Retsevmo
Always take this medicine exactly as your doctor or pharmacist has told you. Check with your doctor or pharmacist if you are not sure.
How much to take
Your doctor will prescribe the right dose for you. The recommended maximum dose is as follows:
- Less than 50 kg body weight: 120 mg twice a day.
- 50 kg body weight or more: 160 mg twice a day.
Retsevmo is taken twice a day, approximately at the same time each day, preferably in the morning and evening.
If you experience any side effects while taking Retsevmo, your doctor may reduce your dose or interrupt treatment temporarily or permanently.
You can take the capsules with or without food. Swallow the capsule whole with a glass of water. Do not chew, crush, or break the capsule before swallowing.
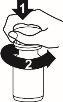
Retsevmo is available in blisters and bottles. The bottle is protected by a plastic screw cap:
To open the bottle, press down on the plastic screw cap while turning it counterclockwise, as shown in the figure.
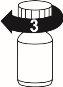
To close the bottle, screw the cap tightly in a clockwise direction.
If you take more Retsevmo than you should
If you take too many capsules, or if someone else takes your medicine, contact a doctor or hospital for advice. You may need medical treatment.
If you forget to take Retsevmo
If you vomit after taking your dose or miss a dose, take your next dose at the usual time. Do not take a double dose to make up for missed or vomited doses.
If you stop taking Retsevmo
Do not stop taking Retsevmo unless your doctor tells you to.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Get medical help immediately if you have any of the following:
- Lung or breathing problems other than lung cancer with symptoms such as difficulty breathing, coughing, and increased temperature (which may affect more than 1 in 10 people)
- Liver problems (which may affect more than 1 in 10 people and may be associated with abnormal blood test results, such as increased liver enzymes) including yellowing of the skin and eyes (jaundice), dark urine, loss of appetite, nausea or vomiting, or pain in the upper right part of your abdomen
- Allergic reactions usually characterized by fever and muscle and joint pain followed by rash (which may affect up to 1 in 10 people)
- High blood pressure (which may affect more than 1 in 10 people)
- Bleeding with symptoms such as coughing up blood
Tell your doctor, pharmacist, or nurse if you notice any of the following side effects:
Very common (may affect more than 1 in 10 people)
- Low calcium levels in the blood
- Reduced number of white blood cells (such as lymphocytes, neutrophils, etc.)
- Low albumin levels in the blood
- Fluid retention that may cause swelling in the hands or ankles (edema)
- Diarrhea
- Increased blood levels of creatinine, which may indicate that the kidneys are not working properly (kidney disorders)
- Fatigue or tiredness
- Dry mouth
- Low sodium levels in the blood
- Reduced number of platelets in the blood, which may cause bleeding and bruising
- Rash
- Abdominal pain
- Constipation
- Low hemoglobin levels, which may cause anemia
- Low magnesium levels in the blood
- Nausea (feeling sick)
- Headache
- Vomiting
- Bleeding symptoms
- Loss of appetite
- Abnormal ECG results
- Low potassium levels in the blood
- Dizziness
- Urinary tract infection
- Fever or high temperature
- Inflammation of the mucous membrane of the mouth
- Decreased thyroid function
Common (may affect up to 1 in 100 people)
- Lymph fluid may accumulate in the lining of the lungs or in the abdominal cavity, which may cause breathing problems or abdominal swelling
- Irregular growth or damage to the hip joint or other bones that causes pain or limping in patients < 18 years of age
Reporting of side effects
If you experience any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly to the Spanish Medicines Agency at www.notificaRAM.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Retsevmo
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the label of the bottle or on the blister and carton after "EXP". The expiry date refers to the last day of the month shown.
This medicine does not require any special storage conditions.
Do not use this medicine if you notice that the inner seal is broken or shows signs of tampering.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help protect the environment.
6. Container Content and Additional Information
Composition of Retsevmo
The active ingredient is selpercatinib. Each hard capsule contains 40 or 80 mg of selpercatinib.
The other components are:
- Capsule content: anhydrous colloidal silica, microcrystalline cellulose
- 40 mg capsule shell: gelatin, titanium dioxide (E171), and iron oxide (E172).
- 80 mg capsule shell: gelatin, titanium dioxide (E171), and brilliant blue FCF (E133).
- Black ink: shellac, ethanol (96%), isopropyl alcohol, butanol, propylene glycol, purified water, concentrated ammonia solution, potassium hydroxide, black iron oxide
Product Appearance and Container Content
Retsevmo 40 mg is supplied as a hard gelatin capsule, gray opaque, with “Lilly”, “3977” and “40 mg” printed in black.
Retsevmo 80 mg is supplied as a hard gelatin capsule, blue opaque, with “Lilly”, “2980” and “80 mg” printed in black.
Retsevmo is available in a white opaque bottle with a plastic screw cap, containing 60 hard capsules of 40 mg and either 60 or 120 hard capsules of 80 mg. Each box contains one bottle.
Retsevmo is presented in blisters of 14, 42, 56, or 168 hard capsules of 40 mg and 14, 28, 56, or 112 hard capsules of 80 mg.
Only some pack sizes may be marketed.
Marketing Authorization Holder
Eli Lilly Nederland B.V., Papendorpseweg 83, 3528BJ Utrecht, Netherlands.
Manufacturer
Lilly S.A., Avda. de la Industria 30, 28108 Alcobendas, Madrid, Spain
For more information about this medicinal product, please contact the local representative of the marketing authorization holder:
Spain
Lilly S.A.
Tel: + 34-91 663 50 00
Date of Last Revision of this Leaflet:July 2024
This medicinal product has been authorized under a “conditional approval”. This approval means that more information on this medicinal product is expected.
The European Medicines Agency will review new information on this medicinal product at least once a year and this leaflet will be updated as necessary.
Other Sources of Information
Detailed information on this medicinal product is available on the European Medicines Agency website: http://www.ema.europa.eu and on the Spanish Agency for Medicines and Health Products (AEMPS) website (http://www.aemps.gob.es/).
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to RETSEVMO 80 mg HARD CAPSULESDosage form: CAPSULE, 40 mgActive substance: selpercatinibManufacturer: Eli Lilly Nederland B.V.Prescription requiredDosage form: TABLET, 100 mgActive substance: avapritinibManufacturer: Blueprint Medicines (Netherlands) B.V.Prescription requiredDosage form: TABLET, 200 mgActive substance: avapritinibManufacturer: Blueprint Medicines (Netherlands) B.V.Prescription required
Online doctors for RETSEVMO 80 mg HARD CAPSULES
Discuss questions about RETSEVMO 80 mg HARD CAPSULES, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions






