
RESPIR 0.5 mg/ml NASAL SPRAY SOLUTION

How to use RESPIR 0.5 mg/ml NASAL SPRAY SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Respir 0.5mg/ml nasal spray solution
oxymetazoline hydrochloride
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Follow exactly the administration instructions of the medicine contained in this leaflet or as indicated by your doctor, pharmacist, or nurse.
- Keep this leaflet, as you may need to read it again.
- If you need advice or more information, consult your pharmacist.
- If you experience side effects, consult your doctor, pharmacist, or nurse, even if they are not listed in this leaflet. See section 4.
- You should consult a doctor if it worsens or does not improve after 3 days.
Contents of the package leaflet:
- What is Respir and what is it used for
- What you need to know before you start using Respir
- How to use Respir
- Possible side effects
- Storage of Respir
- Contents of the pack and further information
1. What is Respir and what is it used for
This medicine belongs to the group of medicines called sympathomimetics. It is a nasal decongestant that contains oxymetazoline as the active ingredient. Oxymetazoline, when administered nasally, produces local vasoconstriction, decongesting the nasal mucosa.
It is indicated for the symptomatic relief of nasal congestion in adults, adolescents, and children from 6 years of age.
You should consult a doctor if it worsens or does not improve after 3 days of treatment.
2. What you need to know before you start using Respir
Do not use Respir
- if you are allergic to oxymetazoline, to other nasal decongestants, or to any of the other components of this medicine (listed in section 6),
- if you have ever suffered from insomnia or dizziness when treated with other sympathomimetic medicines, such as those used to treat heart diseases, hypotension (low blood pressure), or asthma,
- if you are taking monoamine oxidase inhibitors (MAOIs, used to treat Parkinson's disease and depression). MAOIs may increase the hypertensive effects of oxymetazoline,
- if you have narrow-angle glaucoma (increased eye pressure),
- if you have recently undergone head surgery (if you have undergone any cranial, transnasal, or transoral surgical intervention),
- if you have inflammation of the skin and mucosa of the nasal vestibule (anterior and inferior part of the nasal cavities) and crusts in the nose (dry rhinitis),
- if you suffer from acute heart disease or cardiac asthma,
- if you suffer from severe hypertension,
- in children under 6 years of age.
Warnings and precautions
Consult your doctor, pharmacist, or nurse before using this medicine if:
- you have had or currently have any of the following diseases or symptoms:
- high blood sugar levels (diabetes mellitus),
- high blood pressure (arterial hypertension),
- any heart or circulatory disease,
- any prostate disease with difficulty urinating (prostatic hypertrophy),
- any thyroid disease (hyperthyroidism).
In rare cases, oxymetazoline, due to its temporary effects and prolonged use, can increase nasal congestion instead of decreasing it. This is known as rebound effect.
Insomnia can rarely occur after using the medicine. If this happens, avoid using it in the late afternoon or evening.
Do not exceed the recommended dose in section 3. How to use Respir.
To avoid contagion, the medicine should not be used by more than one person, and the applicator should be cleaned after each use with a clean, damp cloth.
Children
Do not use in children under 6 years of age.
Children may be especially prone to the occurrence of adverse effects of this medicine.
Other medicines and Respir
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicine.
This medicine should not be used by people who are taking or have taken during the last 2 weeks:
- medicines used to treat depression (tricyclic antidepressants, maprotiline, or monoamine oxidase inhibitors [MAOIs]) or
- a medicine to lower blood pressure called methyldopa.
It should not be used in case of treatment with phenothiazine (tranquilizer) or with medicines to treat asthma.
This medicine should not be used in conjunction with other vasoconstrictors.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medicine.
Consult your doctor before using this medicine to assess the benefit versus the potential risk.
Driving and using machines
The influence of Respir on the ability to drive and use machines is negligible.
Respir containsbenzalkonium chloride and propylene glycol
This medicine contains 0.2 mg of benzalkonium chloride per milliliter. Each spray of 0.10 ml contains 20 micrograms of benzalkonium chloride. Benzalkonium chloride may cause irritation or inflammation inside the nose, especially when used during long-term treatment.
This medicine contains 100 mg of propylene glycol per milliliter. Each spray of 0.10 ml contains 10 mg of propylene glycol.
3. How to use Respir
Follow exactly the administration instructions of the medicine contained in this leaflet or as indicated by your doctor, pharmacist, or nurse. In case of doubt, ask your doctor, pharmacist, or nurse.
The recommended dose is:
Adults, adolescents, and children over 6 years of age
1 spray in each nostril, every 12 hours, if necessary in the morning and at night.
Do not exceed 2 applications in 24 hours.
Do not repeat at intervals of less than 12 hours.
Consult your doctor if symptoms worsen or do not improve after 3 days of treatment.
Do not exceed the recommended daily dose.
Use in children under 6 years of age
This medicine should not be used in children under 6 years of age (see section 2 Do not use Respir).
Elderly patients (over 65 years of age)
Consult your doctor or pharmacist, as elderly patients are more sensitive to the adverse effects of this medicine.
How to use
This medicine is used nasally.
Before applying this medicine, existing nasal fluids should be eliminated by blowing your nose well.
To avoid contagion, after each use and before closing the container, the applicator tip should be cleaned with a clean, damp cloth. Additionally, each container should be used by only 1 person.
Shake before use.
Nebulizer bottle: with your head in an upright position, place the atomizer opening in the nasal cavity without completely obstructing it. During each application, lean your head slightly forward and inhale quickly while pressing the bottle.
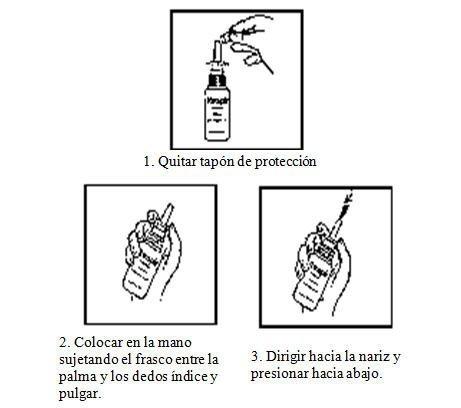
Bottle with spray pump:
Shake well before use. Remove the protective cap.
Before the first use, press the pump several times until the first complete spray is produced.
To spray, hold the bottle between the palm and index finger and thumb. Without tilting your head, insert the nozzle into the nasal cavity.
Press the edge completely with a firm and uniform motion and spray the recommended number of sprays in each nostril. Keep the bottle in a vertical position
See illustrative drawings:
You should consult a doctor if it worsens or does not improve after 3 days of treatment.
If you use more Respir than you should
By applying excessive doses or very continuous ones, or if you accidentally swallow the product, the following symptoms may occur: headache, tremors, insomnia, excessive sweating, palpitations, tachycardia, increased blood pressure, or sleep disturbances.
In children, these effects may include: convulsions and coma, hallucinations, decreased heart rate, temporary suspension of breathing, increased blood pressure that later becomes a decrease in blood pressure, excitability, urticaria, nausea, and vomiting, hysteria, somnolence or drowsiness, abnormal gait, facial edema.
In case of overdose or accidental ingestion, consult your doctor or pharmacist immediately or call the Toxicological Information Service, phone: 91.5620420, indicating the medicine and the amount ingested.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, Respir can cause side effects, although not everybody gets them.
During the period of use of oxymetazoline, the following side effects have been observed, whose frequency could not be established with accuracy.
The most frequent side effect is a sensation of local dryness in the nasal mucosa.
Uncommon side effects are local itching and burning.
The side effects that can occur in rare cases are:
Allergic reaction, nervousness, agitation, anxiety, dizziness or lightheadedness, sleep problems, somnolence, tremors, headache, hallucinations (especially in children), blurred vision, tachycardia, palpitations, increased blood pressure, reactive hyperemia (blood accumulation in an organ due to vein obstruction), nasal burning, sneezing, increased nasal secretion, increased nasal congestion, nasal dryness, dry mouth, nasal irritation, throat irritation, rebound congestion, nausea, rash, convulsions (especially in children), and weakness.
Reporting of side effects:
If you experience any side effect, consult your doctor, pharmacist, or nurse, even if it is a possible side effect not listed in this leaflet. You can also report them directly through the Spanish Pharmacovigilance System for Human Use Medicines: https://www.notificaram.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Respir
Keep this medicine out of the sight and reach of children.
No special storage conditions are required.
Do not use this medicine after the expiry date stated on the bottle after CAD. The expiry date is the last day of the month indicated.
Medicines should not be disposed of via wastewater or household waste. Place the containers and medicines you no longer need in the SIGRE collection point at the pharmacy. If in doubt, ask your pharmacist how to dispose of the containers and medicines you no longer need. This will help protect the environment.
6. Contents of the pack and further information
Composition of Respir
- The active ingredient is oxymetazoline hydrochloride. Each milliliter of nasal spray solution contains 0.5 mg of oxymetazoline hydrochloride
- The other components (excipients) are sodium phosphate monobasic, disodium edetate, propylene glycol (E-1520), benzalkonium chloride, sodium hydroxide or hydrochloric acid for pH adjustment, and purified water.
Appearance of the product and contents of the pack
Respir is a clear and colorless solution.
Respir is presented in:
10 ml polyethylene nebulizer bottle.
10 ml and 20 ml polyethylene bottles with spray pump.
Not all pack sizes may be marketed.
Marketing authorization holder and manufacturer
Marketing authorization holder:
Bayer Hispania, S.L.
Avda. Baix Llobregat, Nº 3 – 5
08970 Sant Joan Despí, Barcelona
Spain
Manufacturer:
Berlimed S.A.
C/ Francisco Alonso nº 7
Polígono Industrial Santa Rosa (Alcalá de Henares)
28806 Madrid
Spain
or
Schering-Plough Labo N.V.
Industriepark, 30
B-2220 Heist-op-den-Berg
Belgium
Date of the last revision of this leaflet:October 2024
Detailed information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
- Country of registration
- Active substance
- Prescription requiredNo
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to RESPIR 0.5 mg/ml NASAL SPRAY SOLUTIONDosage form: NASAL PRODUCT, 0.5 mg/mlActive substance: oxymetazolineManufacturer: Pharmex Advanced Laboratories S.L.Prescription not requiredDosage form: NASAL PRODUCT, 0.5 mg/mlActive substance: oxymetazolineManufacturer: Interpharma S.A.Prescription not requiredDosage form: NASAL PRODUCT, 0.5 mg/mlActive substance: oxymetazolineManufacturer: Laboratorios Vicks S.L.Prescription not required
Online doctors for RESPIR 0.5 mg/ml NASAL SPRAY SOLUTION
Discuss questions about RESPIR 0.5 mg/ml NASAL SPRAY SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions











