
QUINSAIR 240 mg SOLUTION FOR NEBULIZER INHALATION

How to use QUINSAIR 240 mg SOLUTION FOR NEBULIZER INHALATION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Quinsair 240mg solution for inhalation by nebulizer
levofloxacin
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Quinsair and what is it used for
- What you need to know before you use Quinsair
- How to use Quinsair
- Possible side effects
- Storage of Quinsair
- Contents of the pack and other information
1. What is Quinsair and what is it used for
Quinsair contains an antibiotic medicine called levofloxacin. It belongs to a group of antibiotics called fluoroquinolones.
Quinsair is used to treat lung infectionscaused by Pseudomonasaeruginosain adults with cystic fibrosis. It is an antibiotic that is inhaled (breathed) directly into the lungs, where it kills the bacteria that cause the infection. This helps to improve breathing in people with cystic fibrosis.
2. What you need to know before you use Quinsair
Do not use Quinsair:
- if you are allergicto levofloxacin, or to any other quinolone antibiotic, such as moxifloxacin, ciprofloxacin or ofloxacin, or to any of the other ingredients of this medicine (listed in section 6)
- if you have ever had problems with your tendons(such as inflammationor tearof a tendon) during treatment with a quinoloneor fluoroquinolone antibiotic
- if you have epilepsy
- if you are pregnantor breast-feeding
Warnings and precautions
Before you start taking this medicine
You should not take antibacterial medicines that contain fluoroquinolones or quinolones, including Quinsair, if you have experienced any serious side effect with previous use of a quinolone or fluoroquinolone. If this is the case, you should inform your doctor as soon as possible.
During treatment with this medicine
Rarely, pain and swelling of the joints and inflammation or tear of tendons may occur. The risk is greater if you are an elderly person (over 60 years), have received an organ transplant, have kidney problems or are being treated with corticosteroids. Inflammation and tear of tendons can occur within the first 48 hours of treatment and even up to several months after stopping treatment with Quinsair. At the first sign of pain or inflammation of a tendon (for example, in the ankle, wrist, elbow, shoulder or knee), stop taking Quinsair, contact your doctor and rest the affected area. Avoid any unnecessary exercise, as this may increase the risk of tendon tear.
Tell your doctor before you start using Quinsair
if you have or have had any of the following conditions:
- Serious side effects that are long-lasting, disabling and potentially irreversible
Antibacterial medicines that contain fluoroquinolones or quinolones, including Quinsair, have been associated with very rare but serious side effects, some of which were long-lasting (persisting for months or years), disabling or potentially irreversible. These include pain in the tendons, muscles and joints of the upper and lower limbs, difficulty walking, abnormal sensations such as pins and needles, tingling, burning, numbness, pain or itching (paresthesia), sensory disorders such as decreased vision, taste, smell and hearing, depression, decreased memory and concentration, intense fatigue and severe sleep disorders.
If you experience any of these side effects after taking Quinsair, contact your doctor immediately before continuing treatment. You and your doctor will decide whether to continue or stop treatment, also considering the use of an antibiotic of a different class.
- Severe kidney problems.
- A severe allergic reaction. The symptoms are listed in section 4.
- Severe skin reactions
If you are being treated with Quinsair, you may experience a severe skin reaction such as blistering or lesions. Inform your doctor if you notice any skin reaction after using Quinsair.
- Liver problems. The symptoms are listed in section 4,
- Heart rhythm disorders
Quinsair may cause changes in your heart rhythm, especially if you are taking medicines to treat heart problems or have low levels of potassium or magnesium in your blood. Women taking these medicines are more likely to be affected. If you experience palpitations or irregular heartbeat while using Quinsair, you should inform your doctor immediately.
- Seizures and convulsions
Quinolone antibiotics, including Quinsair, may cause seizures or convulsions. If this happens, stop using Quinsair and contact your doctor immediately.
- Depression or mental health problems.
- Nerve damage
Rarely, you may experience symptoms of nerve damage (neuropathy), such as pain, burning, itching, tingling, numbness and/or weakness, especially in the feet and legs or hands and arms. If this happens, stop taking Quinsair and inform your doctor immediately to prevent the development of a potentially irreversible disorder.
- A disease that causes muscle weakness and fatigue called myasthenia gravis.
- Inflammation of a tendon that causes pain, stiffness and/or swelling in the joints (tendinitis)
- If after receiving Quinsair you have experienced difficulty breathing, which can be mild or severe (bronchospasm)
- Expectoration or mucus with blood from the respiratory tract
- Glucose-6-phosphate dehydrogenase deficiency
In patients with glucose-6-phosphate dehydrogenase deficiency (a rare hereditary disease), quinolone antibiotics, such as Quinsair, may cause a predisposition to hematological complications that lead to a sudden increase in body temperature, yellowing of the skin and mucous membranes, dark urine, pallor, fatigue, rapid and heavy breathing and rapid and weak pulse. Consult your doctor if in doubt.
- Diabetes
Quinolone antibiotics, including Quinsair, may cause high or low blood sugar levels. If you are diabetic, you should carefully monitor your blood sugar levels.
- Diarrhea
You may experience diarrhea during or after treatment with Quinsair. If the diarrhea becomes severe or persistent, or if you notice blood in your stool, stop using Quinsair immediately and consult your doctor. Do not take any medicine for diarrhea without consulting your doctor first.
- Antibiotic resistance
Bacteria can become resistant to treatment with an antibiotic over time. This means that Quinsair should not be used to prevent lung infections. It should only be used to treat lung infections caused by Pseudomonasaeruginosa.Consult your doctor if you have any questions or concerns about this.
- Superinfections
Sometimes, prolonged treatment with antibiotics can cause you to contract another infection caused by bacteria that are not affected by the antibiotic (superinfection). Consult your doctor if you have any questions or concerns about superinfection and the use of Quinsair.
- Vision problems
If you notice any change in your vision or any other eye problem while using Quinsair, contact an ophthalmologist immediately.
- Photosensitivity
Quinsair may make your skin more sensitive to sunlight. You should avoid prolonged exposure to sunlight and intense sunlight, and should not use sunbeds or any other UV lamp while using Quinsair and for 48 hours after stopping treatment.
- False test results
Certain tests (e.g. for tuberculosis or to detect strong painkillers) may give false results while you are being treated with Quinsair.
- If you have been diagnosed with an aneurysm of a large blood vessel (aortic aneurysm or large peripheral vessel aneurysm).
- If you have had a previous episode of aortic dissection (tear of the aortic wall).
- If you have been diagnosed with a heart valve problem (regurgitation of the heart valves).
- If you have a family history of aortic aneurysm or aortic dissection, congenital heart valve disease or other risk factors or predisposing conditions (e.g. Marfan syndrome or Ehlers-Danlos vascular syndrome, Turner syndrome or Sjögren's syndrome (an autoimmune inflammatory disease), or vascular disorders such as Takayasu arteritis, giant cell arteritis, Behçet's disease, high blood pressure or atherosclerosis, rheumatoid arthritis (a joint disease) or endocarditis (a heart infection)).
- If you experience severe and sudden pain in the abdomen, chest or back, which can be symptoms of aortic dissection or aneurysm, go to the emergency room immediately. The risk may increase if you are receiving systemic corticosteroid treatment.
- If you start to experience sudden onset of shortness of breath, especially when lying down, or if you notice swelling in the ankles, feet or abdomen or the appearance of heart palpitations (feeling of rapid or irregular heartbeat), you should inform your doctor immediately.
Children and adolescents
Quinsair should not be given to children or adolescents under 18 years of age, as there is not enough information on its use in this age group.
Other medicines and Quinsair
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines. These medicines may interfere with the effects of Quinsair.
Tell your doctorif you are taking any of the following medicines:
- Vitamin K antagonists such as warfarin(used to prevent blood clots). Taking these medicines with Quinsair may increase the risk of bleeding. Your doctor may need to perform regular blood tests to check if your blood is clotting properly.
- Theophylline(used to treat respiratory problems) or non-steroidal anti-inflammatory drugs (NSAIDs) such as fenbufen, acetylsalicylic acid(a active ingredient present in many medicines used to relieve pain and reduce fever, as well as to prevent blood clotting) or ibuprofen. Taking Quinsair at the same time as these medicines may increase the risk of having a seizure (convulsion).
- Medicines such as probenecid(used to prevent gout) or cimetidine(used to treat ulcers). Taking Quinsair at the same time as these medicines may affect how your kidneys process the medicine, which is especially important if you have kidney problems.
- Cyclosporin(used after organ transplants) or medicines that affect heart rhythm(such as antiarrhythmics, tricyclic antidepressants, macrolide antibiotics and antipsychotics). Quinsair may interfere with the effects of these medicines. Your doctor will explain this in more detail.
Pregnancy and breast-feeding
Quinsair should not be used during pregnancy or breast-feeding. If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor for advice before taking this medicine.
Driving and using machines
Quinsair may make you feel dizzy, tired or weak, or cause vision problems. If this happens, do not drive or use tools or machines.
3. How to use Quinsair
Follow your doctor's administration instructions for this medication exactly. If in doubt, consult your doctor again.
How much product should I use?
Inhale the contents of one ampoule (240mg) twice a day using the Zirela nebulizer system.It takes around 5 minutes to inhale the medication with the nebulizer.
When should I use it?
Inhaling Quinsair at the same time every day will help you remember when to take the medication. Inhale the medication as follows:
- 1 ampoule in the morning using the Zirela nebulizer
- 1 ampoule in the evening using the Zirela nebulizer
Ideally, leave an interval of around 12 hours between doses.
For how long should I use it?
You should use Quinsair every day for 28 days and then take a 28-day break during which you will not inhale Quinsair. After that, you will start a new treatment cycle.
It is essential that you continue using the medication twice a day during the 28-day treatment period and maintain the cycle of 28 days with treatment and 28 days without treatment for as long as your doctor indicates.


If I experience breathing difficulties when using Quinsair, what additional medication can my doctor prescribe for me?
If you experience breathing difficulties after using Quinsair, your doctor may prescribe an inhaler containing a bronchodilator medication (e.g., salbutamol). Inhale this medication at least 15 minutes and up to 4 hours before your next dose of Quinsair.
What if I am using several different inhalers and other treatments for cystic fibrosis?
If you are receiving several different inhaled treatments and other treatments for cystic fibrosis, it is recommended that you use the medications in the following order:
1. Bronchodilators
2. Dornase alfa
3. Airway clearance techniques
4. Quinsair
5. Inhaled steroids
How to use it
Quinsair should be administered by inhalation using a Zirela handheld nebulizer(which includes a Zirela aerosol head). This should be connected to either an eBase controller or an eFlow rapid control unit.
Important information to know before starting
- Each ampoule is for single use only. Once theampoule is opened, the contents must be used immediately.
- Do not use Quinsair if you notice that the packaging or ampoules have been tampered with.
- Do not use Quinsair if you notice that it is cloudy or contains particles in the solution.
- Do not mix Quinsair with any other medicationin the Zirela handheld nebulizer.
- Do not introduce any other medication other than Quinsair into the Zirela handheld nebulizer.
- Do not attempt to inhale Quinsair using any other type of handheld nebulizer.
- Check that your Zirela nebulizer system is working correctly before starting your treatment.
- Do not swallow the liquid from the ampoule.
Read the manufacturer's instructions for use that come with your Zirela handheld nebulizer carefully.
How should I prepare my nebulizer system to inhale the medication?
Keep the Zirela instructions in a safe place, as they detail how to assemble the device.
- Make sure to place the Zirela handheld nebulizeron a horizontal and stable surface.
- Insert the entire contents of one ampouleinto the medication reservoir of the Zirela handheld nebulizer (Figure 1). Make sure to empty the ampoule completely, tapping it gently against the side of the reservoir if necessary.
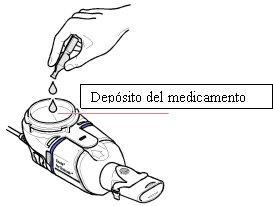
Figure1
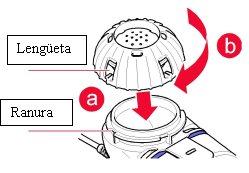 Close the medication reservoirby aligning the medication cap tabs with the reservoir grooves (a). Press and turn the cap clockwise as far as possible (b, Figure 2).
Close the medication reservoirby aligning the medication cap tabs with the reservoir grooves (a). Press and turn the cap clockwise as far as possible (b, Figure 2).
Figure2
How should I use the Zirela nebulizer system?
- To start your treatment,sit in a relaxed and upright position.
- Hold the nebulizer level, press and hold the on/off button on the controller for a few seconds. You will hear a beep and the status light will turn green.
- After a few seconds, an aerosol cloud will start to flowinto the aerosol chamber of the Zirela handheld nebulizer. If the aerosol cloud does not start to flow, try to troubleshoot by consulting the Zirela manufacturer's instructions for use.
- Keeping the nebulizer level, insert the mouthpiece into your mouth and close your lips around it (Figure 3).
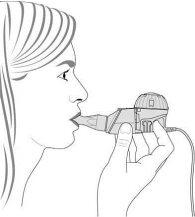
Figure3
- Breathe normally(inhale and exhale) through the mouthpiece. Try not to breathe through your nose. Continue inhaling and exhaling comfortably until the treatment is finished. It takes around 5 minutes to inhale the medication with the nebulizer.
- When all the medication has been released, you will hear two beeps, indicating that the treatment is complete.
- Once completed, open the medication capto ensure that all the medication has been used. You may find a few drops of medication left in the bottom of the reservoir at the end of the treatment. This is normal. However, if there is more than a few drops left, replace the cap and resume treatment.
- Once the treatment is completed, disconnect the controller and disassemble the Zirela handheld nebulizer to clean and disinfect it. The manufacturer's instructions for use provide all the details on cleaning and disinfection.
What if I need to stop the treatment before it's finished?
If for any reason you need to stop the treatment before it's finished, press and hold the on/off button on the controller for one second. Once it's completely off and when you're ready to resume, press and hold the on/off button for one second again. The treatment will resume. Now, inhale and exhale through the mouthpiece as before.
How and when should I replace the Zirela handheld nebulizer?
The Zirela handheld nebulizer should be used for one 28-day treatment cycle. Consult the information on cleaning and storage in the manufacturer's instructions for use.
If you use more Quinsair than you should
If you have used more Quinsair than you should, tell your doctor as soon as possible. You may experience symptoms such as an irregular heartbeat, which your doctor should check. If you swallow the contents of the ampoule, do not worry, but tell your doctor as soon as possible.
If you forget to use Quinsair
If you miss a dose, administer it as soon as you remember, provided that there is an interval of 8 hours until the next dose. If it is almost time for the next dose, skip the missed dose.
Do not inhale the contents of more than one ampoule to make up for the missed dose.
If you stop using Quinsair
Do not stop using Quinsair without consulting your doctor first, as your lung infection may worsen.
If you have any other questions about using this medication, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Some side effects can be serious
If you notice a severe allergic reactionafter inhaling Quinsair, get urgent medical attentionimmediately. The symptoms include:
- Itching and a feeling of heat affecting especially the scalp, mouth, throat, palms of the hands, and soles of the feet.
- Wheezing, whistling sounds when breathing (wheezing), or noisy or difficult breathing
- Hives or severe rash
- Swelling of the lips, face, throat, or tongue
- Pale or grayish skin color
- Fast heartbeat
- Fainting or feeling faint
Stop using Quinsair and contact your doctor immediately:
- if you experience joint pain, stiffness, and/or swelling
- if you have liver problems. The symptoms are:
- Loss of appetite
- Yellowing of the skin and eyes (jaundice)
- Dark-colored urine
- Itching
- Pain around the stomach (abdomen)
Other possible side effects:
Very common: may affect more than 1 in 10 people
- Cough
- Abnormal taste
- Fatigue, weakness, and reduced exercise tolerance
- Loss of appetite and weight loss
- Shortness of breath
- Changes in the amount and density of mucus or phlegm
- Coughing up blood
- Reduced amount of air that can be exhaled in one second (reduced FEV1)
Common: may affect up to 1 in 10 people
- Fungal infection around the vagina
- Insomnia or difficulty sleeping
- Headache
- Dizziness
- Ringing or other sounds in the ears (tinnitus)
- Changes in voice
- Nausea and vomiting
- Abdominal pain
- Diarrhea
- Constipation
- Rash
- Joint or muscle pain
- Fever
- Abnormal blood test results (increased levels of certain liver enzymes or bilirubin, and reduced kidney function test values)
- Reduced lung function test values
- Increased or decreased blood sugar (glucose) levels
- Abnormal breathing sounds
Uncommon: may affect up to 1 in 100 people
- Fungal infection in the mouth
- Reduced number of red blood cells, platelets, or cells in the blood that help it clot
- Reduced or increased number of white blood cells in the blood
- Feeling anxious, restless, or agitated and/or depressed
- Reduced sense of smell
- Drowsiness
- Changes in vision
- Hearing loss
- Increased heart rate
- Difficulty breathing
- Retching
- Indigestion
- Flatulence
- Hives or rash and itching
- Chest pain (thoracic)
- Kidney failure
- Changes in heart rhythm
- Pain, burning, tingling, numbness, or weakness in the limbs (neuropathy)
The following side effects have also been reported after taking tablets or receiving an intravenous infusion containing levofloxacin, so they may occur after using Quinsair:
Uncommon: may affect up to 1 in 100 people
- Feeling confused or nervous
- Tremor
- Feeling dizzy, spinning, or falling (vertigo)
- Excessive sweating
Rare: may affect up to 1 in 1,000 people
- Hallucinations and/or paranoid perceptions
- Feeling agitated
- Unusual dreams or nightmares
- Seizures (fits)
- Tingling, numbness, or pain (neuropathy)
- Palpitations
- Low blood pressure
- Muscle weakness
- Syndrome associated with disturbances in water elimination and low sodium levels (SIADH)
(SIADH)
- Widespread rash, high body temperature, elevated liver enzymes, blood abnormalities (eosinophilia), swollen lymph nodes, and other organs involved (drug reaction with eosinophilia and systemic symptoms)
Rare: may affect up to 1 in 1,000 people
- Red, clearly defined patches with or without blisters
Frequency not known: cannot be estimated from the available data
- Reduced number of all types of blood cells
- Diabetic coma
- Severe mental problems (in very rare cases, can lead to self-harm)
- Pain, burning, tingling, numbness, or weakness in the limbs (neuropathy)
- Involuntary muscle movements, tics, or spasms
- Fainting
- Severe, stabbing headaches with loss of vision
- Temporary loss of vision
- Fast or abnormal heartbeat
- Lung inflammation
- Severe skin reactions such as painful blisters or lesions possibly in the mouth, nose, or vagina
- Increased sensitivity of the skin to sunlight or ultraviolet light (sunbeds or other UV lamps)
- Inflammation of blood vessels
- Inflammation of the mouth or lips
- Rapid muscle breakdown
- Inflammation of a tendon or tendon rupture
- Pain, including back pain, chest pain (thoracic), arm pain, and leg pain
The administration of antibiotics containing quinolones and fluoroquinolones has been associated with very rare cases of long-lasting (even months or years) or permanent side effects, such as tendon inflammation, tendon rupture, joint pain, limb pain, difficulty walking, abnormal sensations (such as pinching, tingling, burning, or numbness), fatigue, reduced memory and concentration, effects on mental health (including sleep disorders, anxiety, panic attacks, depression, and suicidal thoughts), and reduced hearing, vision, taste, and smell, in some cases regardless of the presence of pre-existing risk factors.
There have been reports of increased size and weakening or tearing of the aortic wall (aneurysms and dissections), which could lead to rupture and be fatal, and heart valve problems in patients who have received fluoroquinolones. See also section 2.
Reporting side effects
If you experience any side effects, consult your doctor or pharmacist, even if it is a possible side effect not listed in this leaflet. You can also report them directly through the Spanish Pharmacovigilance System for Human Use https://www.notificaRAM.es. By reporting side effects, you can help provide more information on the safety of this medication.
5. Storage of Quinsair
Keep this medication out of sight and reach of children.
Do not use this medication after the expiration date stated on the ampoule, paper sleeve, and cartons after EXP. The expiration date is the last day of the month indicated.
Each ampoule is for single use only. Once the ampoule is opened, the contents must be used immediately. Any unused product should be discarded. Return unused and unopened ampoules to the paper sleeve to protect them from light.
Store in the original package to protect from light. This medication does not require any special storage temperature.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of the packaging and any unused medication. This will help protect the environment.
6. Package Contents and Additional Information
Quinsair Composition
- The active ingredient is levofloxacin. One ampoule contains levofloxacin hemihydrate equivalent to 240 mg of levofloxacin.
- The other components are magnesium chloride hexahydrate and water for injectable preparations.
Appearance of the Product and Package Contents
Quinsair is a clear, pale yellow solution for inhalation by nebulizer.
The medication comes in small 3 ml plastic ampoules. There are four ampoules sealed in each paper pouch.
Quinsair is supplied in 28-day packages (containing an inner box with 56 (14 pouches of 4) ampoules) or in 2-day packages (containing an inner box with 8 (2 pouches of 4) ampoules) and a box containing a Zirela handheld nebulizer with the manufacturer's instructions for use.
Only some package sizes may be marketed.
The ampoule is only labeled in English. The information that appears on the ampoule is:
On the front of the ampoule end
Quinsair 240 mg
Solution for inhalation by nebulizer
Levofloxacin
Inhalation route 2.4 ml
On the "corrugated area" on both sides of the ampoule end
BATCH
EXP
Marketing Authorization Holder
Chiesi Farmaceutici S.p.A.
Via Palermo, 26/A
43122 Parma
Italy
Manufacturer
Adare Pharmaceuticals S.r.l.
Via Martin Luther King, 13
20060 Pessano con Bornago (MI)
Italy
You can request more information about this medication by contacting the local representative of the marketing authorization holder:
Belgium Chiesi sa/nv Tel: + 32 (0)2 788 42 00 | Lithuania Chiesi Pharmaceuticals GmbH Tel: + 43 1 4073919 |
| Luxembourg Chiesi sa/nv Tel: + 32 (0)2 788 42 00 |
Czech Republic Chiesi CZ s.r.o. Tel: + 420 261221745 | Hungary Chiesi Hungary Kft. Tel.: + 36-1-429 1060 |
Denmark Chiesi Pharma AB Tlf: + 46 8 753 35 20 | Malta Chiesi Farmaceutici S.p.A. Tel: + 39 0521 2791 |
Germany Chiesi GmbH Tel: + 49 40 89724-0 | Netherlands Chiesi Pharmaceuticals B.V. Tel: + 31 88 501 64 00 |
Estonia Chiesi Pharmaceuticals GmbH Tel: + 43 1 4073919 | Norway Chiesi Pharma AB Tlf: + 46 8 753 35 20 |
Greece Chiesi Hellas AEBE Tel: + 30 210 6179763 | Austria Chiesi Pharmaceuticals GmbH Tel: + 43 1 4073919 |
Spain Chiesi España, S.A.U. Tel: + 34 93 494 8000 | Poland Chiesi Poland Sp. z.o.o. Tel.: + 48 22 620 1421 |
France Chiesi S.A.S. Tel: + 33 1 47688899 | Portugal Chiesi Farmaceutici S.p.A. Tel: + 39 0521 2791 |
Croatia Chiesi Pharmaceuticals GmbH Tel: + 43 1 4073919 | Romania Chiesi Romania S.R.L. Tel: + 40 212023642 |
Ireland Chiesi Farmaceutici S.p.A. Tel: + 39 0521 2791 | Slovenia Chiesi Slovenija d.o.o. Tel: + 386-1-43 00 901 |
Iceland Chiesi Pharma AB Tel: +46 8 753 35 20 | Slovakia Chiesi Slovakia s.r.o. Tel: + 421 259300060 |
Italy Chiesi Italia S.p.A. Tel: + 39 0521 279 | Finland Chiesi Pharma AB Tel: +46 8 753 35 20 |
Cyprus Chiesi Farmaceutici S.p.A. Tel: + 39 0521 2791 | Sweden Chiesi Pharma AB Tel: +46 8 753 35 20 |
Latvia Chiesi Pharmaceuticals GmbH Tel: + 43 1 4073919 |
Date of the last revision of this prospectus: April 2025.
Other sources of information
Detailed information about this medication is available on the European Medicines Agency website: https://www.ema.europa.eu. There are also links to other websites about rare diseases and orphan medicines.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to QUINSAIR 240 mg SOLUTION FOR NEBULIZER INHALATIONDosage form: TABLET, 500 mgActive substance: levofloxacinManufacturer: Especialidades Farmaceuticas Centrum S.A.Prescription requiredDosage form: TABLET, 500 mgActive substance: levofloxacinManufacturer: Mabo Farma S.A.Prescription requiredDosage form: TABLET, 500 mgActive substance: levofloxacinManufacturer: Accord Healthcare S.L.U.Prescription required
Online doctors for QUINSAIR 240 mg SOLUTION FOR NEBULIZER INHALATION
Discuss questions about QUINSAIR 240 mg SOLUTION FOR NEBULIZER INHALATION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions
















