
PULMOTEC TM GRAPHITE CRUCIBLE FOR THE PREPARATION OF TECHNEGAS FOR INHALATION

How to use PULMOTEC TM GRAPHITE CRUCIBLE FOR THE PREPARATION OF TECHNEGAS FOR INHALATION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
PULMOTEC Graphite Crucible for the Preparation of Technegas for Inhalation Radiochemical Reagent Preparation Equipment
High-purity graphite
Read this package leaflet carefully before this medicine is administered to you, as it contains important information for you.
- Keep this package leaflet, as you may need to read it again.
- If you have any questions, consult your nuclear medicine doctor, who will supervise the procedure.
- If you experience side effects, consult your nuclear medicine doctor, even if they are side effects not listed in this package leaflet. See section 4.
Contents of the Package Leaflet:
- What PULMOTEC is and what it is used for
- What you need to know before starting to use PULMOTEC
- How to use PULMOTEC
- Possible side effects
- Storage of PULMOTEC
- Package Contents and Additional Information
1. What PULMOTEC is and what it is used for
This medicine is for diagnostic use only.
PULMOTEC, when in the presence of pertechnetate (Tc-99m) sodium and heated to 2550°C in a high-purity argon atmosphere, produces a carbon microparticle aerosol labeled with technetium (Tc-99m), called Technegas.
After inhaling Technegas, lung images can be recorded.
These images will help your doctor or nuclear medicine specialist observe if your lungs are abnormally ventilated. The use of Technegas is usually combined with the injection of another radiopharmaceutical agent into your veins to detect any anomalies in pulmonary blood flow.
2. What you need to know before starting to use PULMOTEC
Warnings and Precautions:
- Technegas is administered by specially trained personnel. Laws governing the use, possession, and handling of radioactive substances stipulate that this medicine can only be used in hospitals or similar institutions.
Your doctor or the nuclear medicine specialist performing the procedure will tell you if you need to take special precautions after using this medicine.
If you have any questions, consult your doctor or the nuclear medicine specialist performing the procedure.
- The use of Technegas involves the administration of a small amount of radioactivity.
The risk associated with its use is very small. Your doctor or nuclear medicine specialist will prescribe this procedure only if they consider the risk to be significantly lower than the potential benefits.
Other Medicines and PULMOTEC:
To date, no interactions with other medicines are known.
Tell your nuclear medicine doctor if you are using or have recently used other medicines, including those purchased without a prescription.
Pregnancy and Breastfeeding:
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your nuclear medicine doctor before using this medicine.
It is essential that you inform your doctor or nuclear medicine specialist if you are pregnant or breastfeeding.
If you are pregnant:
The use of radiopharmaceuticals during pregnancy requires very special care.
Your doctor or nuclear medicine specialist will prescribe this procedure only if they consider the benefits to outweigh the potential risk.
If you are breastfeeding:
If the use of Technegas is considered essential while you are breastfeeding, your doctor or nuclear medicine specialist will ask you not to feed your child for 12 hours after use and to discard the milk produced during this time.
Driving and Using Machines
No studies have been conducted on the effect of this medicinal product on the ability to drive or use machines.
3. How to Use PULMOTEC
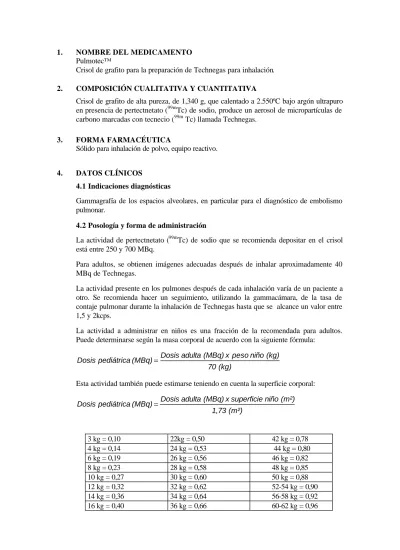
Your doctor or nuclear medicine specialist will know how much Technegas you need to use to achieve an image that provides the necessary medical information. For adults, the usual inhaled dose is around 40 MBq.
The becquerel (Bq) is a unit of radioactivity. MBq means megabecquerel.
Lower doses are used for children.
- Technegas is inhaled. Since the first inhalation does not contain oxygen, you will be given oxygen for a few moments before inhaling Technegas. There are several possible ways to use this product: to determine which is the best way for you, you will be asked to try breathing through the mouthpiece without Technegas and then repeat it with the mouthpiece connected to the Technegas generator.
- If you feel uncomfortable using the medicinal product, you can remove the mouthpiece from your mouth between two Technegas inhalations.
- To achieve a homogeneous distribution of the medicinal product in your lungs, you may need to use Technegas while lying down.
- A series of 4 to 6 images is usually sufficient for your doctor or nuclear medicine specialist to obtain the necessary information.
If you use more PULMOTEC than you should:
- An overdose is virtually impossible. Doses are carefully prepared and verified.
- If an overdose is suspected, your doctor or nuclear medicine specialist will ask you to drink plenty of fluids and urinate frequently.
If you have any other questions about using this medicine, ask your doctor or the nuclear medicine specialist performing the procedure.
4. Possible Side Effects
Like all medicines, this medicine can cause side effects, although not everyone gets them.
The frequencies of side effects are defined as follows: | |
Very common | Affects more than 1 in 10 users |
Common | Affects 1-10 in 100 users |
Uncommon | Affects 1-10 in 1,000 users |
Rare | Affects 1-10 in 10,000 users |
Very rare | Affects less than 1 user in 10,000 |
Not known | Frequency cannot be estimated from the available data |
- Rare cases of dizziness, drowsiness, and nausea have been reported. They are believed to be related to a temporary decrease in oxygen in the blood, produced in the first inhalation of Technegas, which does not contain oxygen. This risk is minimized by administering oxygen before inhaling Technegas.
- If you experience these effects, the doctor or nuclear medicine specialist will let you breathe normal air or administer oxygen.
Reporting Side Effects
If you experience any side effects, consult your doctor, pharmacist, or nurse, even if they are possible side effects not listed in this package leaflet. You can also report them directly through the Spanish Medicines and Healthcare Products Agency (AEMPS) website: www.notificaRAM.es
By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of PULMOTEC
You will not need to obtain or store this medicine for diagnostic use. The center where the test is performed will do so.
The medicine label states the suitable storage conditions and expiration date. Hospital personnel will verify that the medicine is stored under the established conditions and not used after its expiration date.
6. Package Contents and Additional Information
PULMOTEC Composition:
- The active substance is high-purity graphite (99.9%): 1.340 g in each crucible
- There are no other excipients.
Appearance of the Product and Package Contents:
Radiochemical reagent preparation equipment.
Technegas is obtained by heating the radiopharmaceutical agent pertechnetate (99mTc) sodium to 2550°C in a high-purity graphite crucible. Technegas is a carbon microparticle aerosol suspended in argon gas.
PULMOTEC crucibles of 135 µL
Five shrink-wrapped (PVC-cardboard) packages with 10 crucibles of 135 µL per package of PULMOTEC in a cardboard box.
PULMOTEC crucibles of 300 µL
Five shrink-wrapped (PVC-cardboard) packages with 10 crucibles of 300 µL per package of PULMOTEC in a cardboard box.
Marketing Authorization Holder and Manufacturer:
Marketing Authorization Holder
CYCLOMEDICA IRELAND LTD
Unit A5 Calmount Business Park
Ballymount
Dublin 12
IRELAND
Manufacturer
Pharmapac Limited
Unit D1
Willow Drive
Naas Enterprise Park
Newhall
Naas
Co. Kildare
W91 E797
Ireland
Date of Last Revision of this Package Leaflet12/2024
Detailed and updated information on this medicine is available on the website of the Spanish Agency for Medicines and Healthcare Products (AEMPS) http://www.aemps.es/
This information is intended only for healthcare professionals:
Please consult the summary of product characteristics.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to PULMOTEC TM GRAPHITE CRUCIBLE FOR THE PREPARATION OF TECHNEGAS FOR INHALATIONDosage form: RADIOPHARMACEUTICAL, 2 mgActive substance: technetium (99mTc) macrosalbManufacturer: Cis Bio InternationalPrescription requiredDosage form: RADIOPHARMACEUTICAL, 2.5 mgActive substance: technetium (99mTc) macrosalbManufacturer: Medi-Radiopharma Kft.Prescription requiredDosage form: INJECTABLE, 74 MBq iobenguane (123I)Active substance: iobenguane (123I)Manufacturer: Ge Healthcare Bio-Sciences, S.A.U.Prescription required
Online doctors for PULMOTEC TM GRAPHITE CRUCIBLE FOR THE PREPARATION OF TECHNEGAS FOR INHALATION
Discuss questions about PULMOTEC TM GRAPHITE CRUCIBLE FOR THE PREPARATION OF TECHNEGAS FOR INHALATION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions












