
PROSPANTUS JARABE EN SOBRES

How to use PROSPANTUS JARABE EN SOBRES
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Leaflet: information for the user
Prospantus Syrup in sachets
Dry extract of Hedera helix L. (ivy)
Read the entire leaflet carefully before starting to take this medication because it contains important information for you.
Follow exactly the administration instructions of the medication contained in this leaflet or as indicated by your doctor or pharmacist.
- Keep this leaflet, as you may need to read it again.
- If you need advice or more information, consult your pharmacist.
- If you experience side effects, consult your doctor or pharmacist, even if they are side effects not listed in this leaflet. See section 4.
- You should consult a doctor if it worsens or does not improve after 7 days.
Contents of the leaflet
- What Prospantus Syrup in sachets is and what it is used for
- What you need to know before taking Prospantus Syrup in sachets
- How to take Prospantus Syrup in sachets
- Possible side effects
- Storage of Prospantus Syrup in sachets
- Package contents and additional information
1. What Prospantus Syrup in sachets is and what it is used for
Prospantus Syrup in sachets is an expectorant.
Prospantus Syrup in sachets is a herbal medicine used as an expectorant for productive cough accompanying benign bronchial conditions. It facilitates the elimination of mucus.
Prospantus Syrup in sachets is indicated for adults, adolescents, and children over 6 years of age.
2. What you need to know before taking Prospantus Syrup in sachets
Do not take Prospantus Syrup in sachets:
- If you are allergic to ivy (Hedera helix L.), to plants of the Araliaceae family, or to any of the other components of this medication (listed in section 6).
- Do not administer to children under 2 years of age, as there is a risk of worsening respiratory symptoms.
Warnings and precautions:
Consult your doctor or pharmacist before starting to take Prospantus Syrup in sachets.
You should consult with your doctor or pharmacist in cases of dyspnea (difficulty breathing), fever, or purulent sputum.
Concomitant use with other antitussives such as codeine or dextromethorphan is not recommended without prior medical consultation.
Cautious use is recommended in patients with gastritis or gastric ulcer.
This medication should not be administered to children from 2 to 5 years of age, as it cannot be dosed adequately.
In case of worsening symptoms or if no improvement occurs after 7 days of starting treatment, treatment should be discontinued and a doctor consulted.
Taking Prospantus Syrup in sachets with other medications:
Inform your doctor or pharmacist if you are taking, have recently taken, or may need to take any other medication.
Pregnancy, breastfeeding, and fertility:
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medication.
Pregnancy:
There are no adequate and well-controlled studies in pregnant women, so its administration is not recommended.
Breastfeeding:
There is no information on the passage of the components of this medication into breast milk, so its administration is not recommended in breastfeeding women.
Consult your doctor or pharmacist before using any medication.
Driving and using machines
No studies have been conducted on the effects on the ability to drive and use machines.
Prospantus Syrup in sachets contains sorbitol.If your doctor has told you that you have an intolerance to certain sugars, consult with them before taking this medication.
3. How to take Prospantus Syrup in sachets
Follow exactly the administration instructions of this medication contained in this leaflet or as indicated by your doctor or pharmacist. In case of doubt, ask your doctor or pharmacist.
The recommended dose is:
- Adults and adolescents over 12 years:5 ml of syrup (1 sachet), 3 times a day, (equivalent to 105 mg daily of dry extract of ivy leaves).
- Children between 6 and 12 years of age:5 ml of syrup (1 sachet), 2 times a day, (equivalent to 70 mg daily of dry extract of ivy leaves).
If you think the action of Prospantus Syrup in sachets is too strong or too weak, inform your doctor or pharmacist.
Prospantus Syrup in sachets is taken orally.
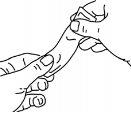
For more details on the use of the sachets, follow the diagrams below:
Press the sachet gently before using it, as shown.
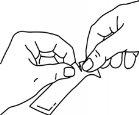
Hold the sachet firmly and tear it along the indicated lines.
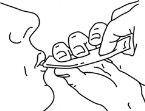
Swallow the medication, squeezing the sachet until it is empty.
You should consult a doctor if it worsens or does not improve after a week of treatment.
If you take more Prospantus Syrup in sachets than you should
In case you have taken more Prospantus Syrup in sachets than you should, or in case of overdose or accidental ingestion, consult your doctor or pharmacist immediately or call the Toxicology Information Service, phone 915 620 420, indicating the medication and the amount ingested.
Do not exceed the recommended daily dose. Ingestion of significantly higher amounts (more than three times the daily dose) may cause nausea, vomiting, and diarrhea.
In this case, you should consult your doctor.
If you forget to take Prospantus Syrup in sachets:
Do not take a double dose to make up for the forgotten dose.
If you have any other doubts about the use of this medication, ask your doctor or pharmacist.
4. Possible side effects
Like all medications, this medication Prospantus may produce side effects, although not all people experience them.
Frequent (may affect 1 to 10 out of 100 patients): gastrointestinal system reactions such as nausea, vomiting, or diarrhea have been reported.
Uncommon (may affect 1 to 10 out of 1000 patients): allergic reactions such as urticaria, skin rashes, difficulty breathing (dyspnea) have been reported.
If you experience side effects, consult your doctor or pharmacist, even if they are side effects not listed in this leaflet.
If you notice symptoms of allergy (hypersensitivity), discontinue the use of Prospantus Syrup in sachets.
Reporting side effects
If you experience any type of side effect, consult your doctor or pharmacist, even if it is a possible side effect not listed in this leaflet. You can also report them directly through the Spanish Medicines and Healthcare Products Agency (AEMPS) website: https://www.notificaram.es. By reporting side effects, you can contribute to providing more information on the safety of this medication.
5. Storage of Prospantus Syrup in sachets
Keep this medication out of sight and reach of children.
No special storage conditions are required.
Do not use this medication after the expiration date shown on the packaging, after "EXP". The expiration date is the last day of the month indicated.
Medications should not be disposed of through wastewater or household waste. Deposit the packaging and medications you no longer need at the pharmacy's SIGRE point. If in doubt, ask your pharmacist how to dispose of the packaging and medications you no longer need. This way, you will help protect the environment.
6. Package contents and additional information
Composition of Prospantus Syrup in sachets:
- The active ingredient is: dry extract of ivy leaf. 5 ml of Prospantus Syrup in sachets contain 35 mg of dry extract of Hedera helixL., leaf (5-7.5:1), extraction solvent: ethanol 30% m/m.
- The other components are: purified water, potassium sorbate, anhydrous citric acid, crystallizable sorbitol liquid, xanthan gum, and cherry flavor.
Appearance of the product and package contents:
Prospantus Syrup in sachets is presented in sachets containing 5 ml of syrup. The box is 21 sachets of polyethylene terephthalate / adhesive / aluminum / polyethylene complex.
Marketing authorization holder and manufacturer:
Engelhard Arzneimittel GmbH & Co. KG
Herzbergstrasse 3
D-61138 Niederdorfelden
Germany
You can request more information about this medication by contacting the local representative of the marketing authorization holder:
Ferrer Internacional, S.A.
Gran Vía Carlos III, 94
08028 Barcelona
Date of the last revision of this leaflet: April 2017.
Detailed information about this medication is available on the website of the Spanish Agency for Medicines and Healthcare Products (AEMPS) http://www.aemps.gob.es/
- Country of registration
- Active substance
- Prescription requiredNo
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to PROSPANTUS JARABE EN SOBRESDosage form: ORAL SOLUTION/SUSPENSION, 7 mg/mlActive substance: Hederae helicis foliumManufacturer: Engelhard Arzneimittel Gmbh & Co. KgPrescription not requiredDosage form: ORAL SOLUTION/SUSPENSION, 35 mgActive substance: Hederae helicis foliumManufacturer: Adventia Pharma S.L.Prescription not requiredDosage form: ORAL SOLUTION/SUSPENSION, 7 mg/mlActive substance: Hederae helicis foliumManufacturer: Adventia Pharma S.L.Prescription not required
Online doctors for PROSPANTUS JARABE EN SOBRES
Discuss questions about PROSPANTUS JARABE EN SOBRES, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















