PRIMOVIST 0.25 mmol/ml, PRE-FILLED SYRINGE SOLUTION FOR INJECTION

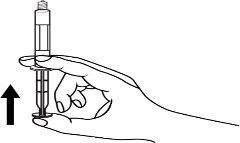
How to use PRIMOVIST 0.25 mmol/ml, PRE-FILLED SYRINGE SOLUTION FOR INJECTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Primovist 0.25 mmol/ml, Solution for Injection in Pre-filled Syringe
Gadolinium Disodium
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- If you get any side effects, talk to your doctor. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack:
- What is Primovist and what is it used for
- What you need to know before you use Primovist
- How to use Primovist
- Possible side effects
- Storage of Primovist
- Contents of the pack and other information
1. What is Primovist and what is it used for
Primovist is a contrast medium for diagnostic imaging by magnetic resonance (MR) of the liver. It is used to help detect and diagnose abnormalities that may appear in the liver. It can better assess abnormal signs of the liver in terms of number, size, and distribution. Primovist can also help your doctor determine the nature of any abnormality, thereby increasing confidence in the diagnosis. It is supplied as an injectable solution. This medicine is for diagnostic use only.
Magnetic Resonance Imaging (MRI) is a diagnostic imaging method that creates images by detecting water molecules in normal and abnormal tissues. This is done using a complex system of magnets and radio waves.
2. What you need to know before you use Primovist
Do not usePrimovist
- If you are allergic to gadolinium disodium or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Talk to your doctor before you start using Primovist if you:
- have or have had asthma or an allergy like hay fever, hives.
- have previously had a reaction to contrast media.
- have reduced kidney function.
The use of some gadolinium-containing contrast agents in patients with this disease has been associated with a condition called Nephrogenic Systemic Fibrosis (NSF). NSF is a disease that leads to thickening of the skin and connective tissues. NSF can cause decreased mobility of joints, muscle weakness, or altered function of internal organs, which can be life-threatening.
- have severe heart or blood vessel disease.
- have low potassium levels in the blood.
- or someone in your family has had a heart rhythm problem called long QT syndrome.
- have had heart rhythm disturbances due to any medication.
- have a heart pacemaker or if there is any implant or clip containing iron in your body.
Delayed allergic reactions may occur hours or days after administration of Primovist. See section 4.
Tell your doctor if:
- your kidneys are not working properly
- you have recently had, or are going to have, a liver transplant
Your doctor may decide to perform a blood test to check the proper functioning of your kidneys before deciding on the use of Primovist, especially if you are 65 years or older.
Accumulation in the body
Primovist works because it contains a metal called gadolinium. Studies have shown that small amounts of gadolinium can accumulate in the body, including the brain.
No adverse effects due to the accumulation of gadolinium in the brain have been observed.
Children and adolescents
The safety and efficacy of Primovist in patients under 18 years have not been established, as experience with this use is limited. Additional information is provided at the end of the leaflet.
Using Primovist with other medicines
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicines. This includes:
- beta blockers, medicines for the treatment of high blood pressure or other heart diseases
- medicines that alter the rhythm or rate of heartbeats, such as amiodarone, sotalol
- rifampicin, a medicine used for the treatment of tuberculosis or other infections.
Pregnancy and breast-feeding
Pregnancy
Gadolinium may cross the placenta. It is not known if it affects the fetus. You should tell your doctor if you are pregnant or think you might be, as Primovist should not be used during pregnancy unless it is strictly necessary.
Breast-feeding
Tell your doctor if you are breast-feeding or about to start. Your doctor will assess whether you should continue breast-feeding or interrupt it for a period of 24 hours after administration of Primovist.
Driving and using machines
Primovist has no influence on the ability to drive and use machines.
Primovist contains sodium
This medicine contains 82 mg of sodium (the main component of cooking/table salt) per dose based on the amount administered for a 70 kg person. This is equivalent to 4.1% of the maximum recommended daily intake of sodium for an adult.
3. How to use Primovist
Primovist is injected into a vein using a small needle. You will be given Primovist immediately before your MRI scan.
After injection, you will be under observation for at least 30 minutes.
Recommended dose
0.1 ml of Primovist per kilogram of body weight.
Dosage in special populations
The use of Primovist is not recommended in patients with severe kidney problems or in patients who have recently had or are going to have a liver transplant. However, if use is required, during an examination, only one dose of Primovist should be administered and a second injection should not be given until at least 7 days have passed.
Elderly patients
If you are 65 years or older, it is not necessary to adjust the dose, but your doctor may perform a blood test to check the proper functioning of your kidneys.
If you use more Primovist than you should:
Overdose is unlikely. If this happens, your doctor will treat any symptoms that appear.
If you have any further questions on the use of this product, ask your doctor or pharmacist.
You can also contact the Toxicology Information Service. Telephone: 91 562 04 20.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Most side effects are mild to moderate.
If you experience any side effects, talk to your doctor.
As with other contrast media, rare cases of allergic reactions may occur. Delayed reactions may occur hours to days after administration of Primovist.
The most serious side effect in patients receiving Primovist is anaphylactic shock (a severe allergic reaction).
Tell your doctor immediately if you experience any of the following signs or if you have difficulty breathing:
- low blood pressure
- swelling of the tongue, throat, or face
- nasal discharge, sneezing, coughing
- red, itchy, watery eyes
- stomach pain
- hives
- decreased sensation or sensitivity in the skin, itching, pale skin
The following additional side effects may occur:
Common side effects:may affect up to 1 in 10 people
- headache
- nausea
Uncommon side effects:may affect up to 1 in 100 people
- dizziness
- numbness and tingling
- alterations in taste or smell
- flushing
- increased blood pressure
- breathing difficulties
- vomiting
- dry mouth
- skin rash
- intense itching, affecting the whole body or eyes
- back pain, chest pain
- reactions at the injection site, such as burning, coldness, irritation, pain
- feeling of heat
- chills
- fatigue
- abnormal sensitivity
Rare side effects:may affect up to 1 in 1,000 people
- inability to sit or stand
- uncontrollable trembling
- abnormally intense or rapid heartbeats (palpitations)
- irregular heartbeats (signs of heart block)
- mouth discomfort
- increased saliva production
- redness of the skin with blisters and spots
- increased sweating
- feeling of discomfort, general malaise
Frequency not known:frequency cannot be estimated from the available data
- rapid heart rate
- agitation
Some laboratory values may change shortly after you have been given Primovist. Tell your healthcare professional if you have had a Primovist test recently, in case they need to perform a blood or urine test.
There have been reports of Nephrogenic Systemic Fibrosis (which causes hardening of the skin and may affect soft tissues and internal organs) associated with the use of other gadolinium-containing contrast agents.
Reporting of side effectsIf you experience any side effects, talk to your doctor, even if it is possible side effects not listed in this leaflet. You can also report side effects directly through the Spanish Pharmacovigilance System for Human Use Medicines: https://www.notificaram.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Primovist
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the label of the syringe or carton after EXP. The expiry date is the last day of the month shown.
This medicine does not require any special storage conditions.
This medicine should be used immediately after opening.
It should be inspected visually before use. This medicine should not be used if it shows significant color changes, appearance of particles, or if the container is defective.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Package Contents and Additional Information
Primovist Composition
- The active ingredient is disodium gadoxetate. Each ml of solution for injection contains 0.25 mmol of disodium gadoxetate (equivalent to 181.43 mg of disodium gadoxetate).
- The other components are: trisodium caloxetate, trometamol, sodium hydroxide, and hydrochloric acid (both for pH adjustment), and water for injectable preparations.
1 pre-filled syringe with 5.0 ml of solution contains 907 mg of disodium gadoxetate,
1 pre-filled syringe with 7.5 ml of solution contains 1361 mg of disodium gadoxetate, [only the glass syringe]
1 pre-filled syringe with 10.0 ml of solution contains 1814 mg of disodium gadoxetate.
Product Appearance and Package Contents
Primovist is a clear, colorless to pale yellow solution free of visible particles. The package contents are 1, 5, or 10 pre-filled syringes with:
5.0 ml of solution for injection (in a 10 ml glass or plastic pre-filled syringe)
7.5 ml of solution for injection (in a 10 ml glass pre-filled syringe) [only the glass syringe]
10.0 ml of solution for injection (in a 10 ml glass or plastic pre-filled syringe)
Only some package sizes may be marketed.
Marketing Authorization Holder:
Bayer Hispania, S.L.
Avda. Baix Llobregat, 3-5
08970 Sant Joan Despí (Barcelona)
Spain
Manufacturer:
Bayer AG
Müllerstrasse 178
13353 Berlin, Germany
Date of Last Revision of this Leaflet: December 2024
Detailed information on this medicinal product is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
-----------------------------------------------------------------------------------------------------------------------------
This information is intended only for healthcare professionals:
- Renal Insufficiency
Before administration of Primovist, it is recommended to evaluate all patients for possible renal dysfunction through laboratory tests.
Cases of nephrogenic systemic fibrosis (NSF) associated with the use of some gadolinium-based contrast agents have been reported in patients with severe acute or chronic renal insufficiency (GFR or glomerular filtration rate <30 ml min 1.73 m2). patients undergoing liver transplantation have a special risk since the incidence of renal failure is high in this group. there possibility that nsf may occur with primovist, it should be avoided:< p>
- in patients with severe renal insufficiency
- during the perioperative period of a liver transplant
unless the diagnostic information is essential and cannot be obtained through contrast-free magnetic resonance imaging (MRI). If the use of Primovist cannot be avoided, the dose should not exceed 0.025 mmol/kg of body weight. During an examination, no more than one dose should be administered. Due to the lack of information on repeated administration, Primovist should not be repeated unless at least 7 days have passed between injections.
Since the renal elimination of gadoxetate may be reduced in elderly patients, it is especially important to evaluate patients aged 65 and older for possible renal dysfunction.
Hemodialysis shortly after administration of Primovist may be useful for the elimination of Primovist from the body. There is no evidence to support the initiation of hemodialysis for the prevention or treatment of NSF in patients who are not undergoing hemodialysis.
- Pregnancy and Lactation
Primovist should not be used during pregnancy unless the clinical situation of the woman requires treatment with gadoxetate.
The continuation or interruption of breastfeeding 24 hours after administration of Primovist will be at the discretion of the doctor and the breastfeeding mother.
- Pediatric Population
An observational study was conducted in 52 pediatric patients aged over 2 months and under 18 years. The patients were referred for a hepatic Magnetic Resonance Imaging (MRI) scan with Primovist enhancement to evaluate known or suspected hepatic focal lesions. Additional diagnostic information was obtained when the hepatic MRI with and without enhancement was compared to the non-enhanced MRI. Serious adverse events were reported, but none of them were related to Primovist by the investigator. Due to the retrospective nature and small sample size of this study, no definitive conclusion can be drawn regarding efficacy and safety in this population.
- Before Injection
Primovist is a clear, colorless to pale yellow liquid free of visible particles. The contrast agent should be visually inspected before use. The contrast agent should not be used if it shows significant color changes, particle formation, or if the package is defective.
- Administration
Primovist should be administered undiluted as an intravenous bolus injection at a flow rate of approximately 2 ml/s. After injection of the contrast agent, the cannula/intravenous line should be flushed with a physiological saline solution (9 mg/ml).
- The patients should be kept under observation for at least 30 minutes after injection.
- Primovist should not be mixed with other medications.
- Intramuscular injection should be strictly avoided.
- Handling
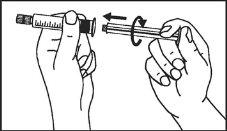
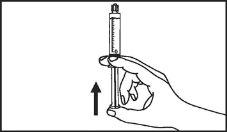
Primovist is ready for use.
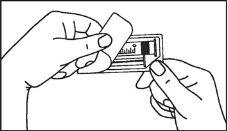
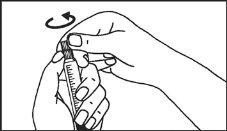
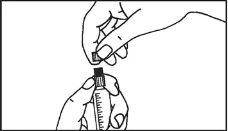
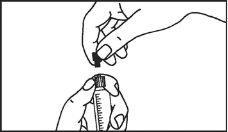
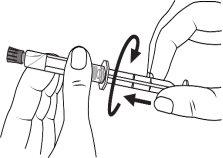
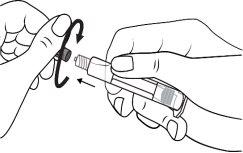
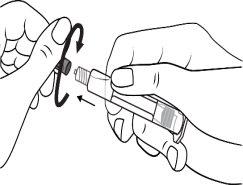
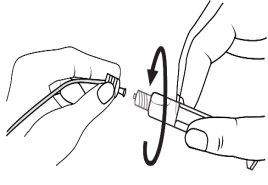
The pre-filled syringe should be prepared for injection immediately before the examination. The cap (capuchon) of the pre-filled syringe should be removed immediately before use.
Any unused contrast agent should be discarded according to local regulations.
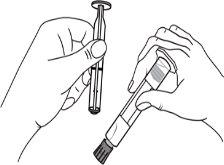
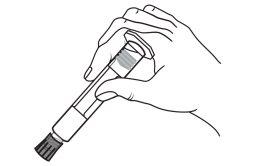
The detachable label of the pre-filled syringes should be stuck to the patient's medical record to allow accurate recording of the gadolinium-based contrast agent used. The dose used should also be recorded. If electronic patient records are used, the product name, batch number, and administered dose should be entered.
Glass syringe:
|
| |
|
| |
|
| |
|
| |
|
| |
|
|
Plastic syringe:
Manual injection | Injection with injector | ||
|
| ||
|
| ||
|
| ||
|
|
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to PRIMOVIST 0.25 mmol/ml, PRE-FILLED SYRINGE SOLUTION FOR INJECTIONDosage form: INJECTABLE, 0.5 mmol/mlActive substance: gadoteric acidManufacturer: Ge Healthcare Bio-Sciences, S.A.U.Prescription requiredDosage form: INJECTABLE, 0.5 mmol/mlActive substance: gadoteric acidManufacturer: Ge Healthcare Bio-Sciences, S.A.U.Prescription requiredDosage form: INJECTABLE, 0.5 mmol/mlActive substance: gadoteric acidManufacturer: Sanochemia Pharmazeutika GmbhPrescription required
Online doctors for PRIMOVIST 0.25 mmol/ml, PRE-FILLED SYRINGE SOLUTION FOR INJECTION
Discuss questions about PRIMOVIST 0.25 mmol/ml, PRE-FILLED SYRINGE SOLUTION FOR INJECTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions