PALYNZIQ 10 mg Injectable Solution in Pre-filled Syringe

How to use PALYNZIQ 10 mg Injectable Solution in Pre-filled Syringe
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Palynziq 2.5 mg solution for injection in pre-filled syringe
Palynziq 10 mg solution for injection in pre-filled syringe
Palynziq 20 mg solution for injection in pre-filled syringe
pegvaliase
This medicine is subject to additional monitoring, which will allow for quick identification of new safety information. You can help by reporting any side effects you may get. The last section of the package leaflet contains information on how to report side effects.
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Palynziq and what is it used for
- What you need to know before you use Palynziq
- How to use Palynziq
- Possible side effects
- Storage of Palynziq
- Contents of the pack and other information
1. What is Palynziq and what is it used for
Palynziq contains the active substance pegvaliase, an enzyme that can break down a substance called phenylalanine in the body. Palynziq is a treatment for patients over 16 years of age who have phenylketonuria (PKU), a very rare inherited disorder that causes phenylalanine from the proteins in food to build up in the body. People with PKU have high levels of phenylalanine, which can cause serious health problems. Palynziq reduces phenylalanine levels in the blood of patients with PKU whose blood phenylalanine levels cannot be controlled below 600 micromol/l by other means, such as diet.
2. What you need to know before you use Palynziq
Do not use Palynziq
- if you are allergic to pegvaliase or any of the other ingredients of this medicine, or to other medicines that contain polyethylene glycol (PEG) (including those listed in section 6).
Warnings and precautions
Talk to your doctor, pharmacist, or nurse before you start using Palynziq.
Allergic reactions
You may have allergic reactions during treatment with Palynziq. Your doctor will explain how to manage allergic reactions according to their severity and will prescribe other medicines to treat the reaction.
Before using Palynziq, tell your doctor if you cannot or do not want to use an adrenaline injection device to treat severe allergic reactions to Palynziq.
Palynziq may cause severe, life-threatening allergic reactionsat any time after receiving the injection.
- Stop injecting Palynziq if you experience any of the following symptoms:
- swelling of the face, eyes, lips, mouth, throat, tongue, hands, or feet;
- difficulty breathing or wheezing;
- a feeling of tightness in the throat or a feeling of choking;
- difficulty swallowing or speaking;
- a feeling of dizziness or fainting;
- loss of control of urine or stool;
- rapid heartbeat;
- hives (a raised, itchy rash) that spread quickly;
- redness;
- severe stomach pain, vomiting, or diarrhea.
- Use the adrenaline injection device according to the doctor's instructions and seek urgent medical help.
Your doctor will prescribe an adrenaline injection device to treat severe allergic reactions and will explain to you and your caregiver when and how to administer adrenaline. Always carry the adrenaline injection device with you.
For at least the first 6 months of treatment, someone should be with youwhen you self-inject Palynziq. Your companion should stay with you for at least 1 hour after the injection to observe for any signs or symptoms of a severe allergic reaction and, if necessary, administer an adrenaline injection and seek urgent medical help.
If you have a severe allergic reaction, do not continue using Palynziquntil you have talked to the doctor who prescribed Palynziq. Tell the doctor that you have had a severe allergic reaction. The doctor will tell you whether you can continue treatment with Palynziq.
Time needed to lower blood phenylalanine levels
When starting treatment with Palynziq, your doctor will prescribe a low dose, which will be gradually increased. It will take some time to find the most effective dose to reduce your blood phenylalanine levels. Most people respond within 18 months, but it may take up to 30 months.
Injecting other PEG-containing medicines while using Palynziq
Palynziq contains an ingredient called polyethylene glycol (PEG). If you inject Palynziq with another injectable medicine that contains PEG, such as pegylated medroxyprogesterone acetate, you may have an allergic reaction. Tell your doctor or pharmacist if you are injecting, have recently injected, or may need to inject any other medicine.
Low blood phenylalanine levels
When using Palynziq, you may have low blood phenylalanine levels. Your doctor will check your blood phenylalanine levels monthly. If they are too low, you may be asked to make changes to your diet and/or reduce the dose of Palynziq. Your doctor will check your blood phenylalanine levels every 2 weeks until they return to normal.
Children and adolescents
It is not known if Palynziq is safe in children and adolescents under 16 years of age who have PKU, and therefore it should not be used in people under 16 years of age.
Other medicines and Palynziq
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicines.
Pregnancy, breastfeeding, and fertility
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctor for advice before taking this medicine.
Palynziq should not be used during pregnancy unless necessary to treat your condition and other methods to control blood phenylalanine levels are not effective. If your blood phenylalanine levels are too high or too low during pregnancy, it may be harmful to you or your baby. You and your doctor will decide the best way to control your blood phenylalanine levels before and during pregnancy.
It is not known if Palynziq passes into breast milk or if it will affect your baby. Ask your healthcare professional for advice on the best way to feed your baby if you use Palynziq.
It is not known if Palynziq affects fertility. Animal studies suggest that women may have difficulty getting pregnant if their blood phenylalanine levels are too low.
Driving and using machines
Palynziq may affect your ability to drive and use machines if you have a severe allergic reaction.
Palynziq contains sodium
This medicine contains less than 1 mmol of sodium (23 mg) per pre-filled syringe; this is essentially “sodium-free”.
3. How to use Palynziq
Follow the doctor's instructions for administering this medicine exactly.
In case of doubt, consult your doctor again.
Palynziq is administered by injection under the skin (subcutaneous injection).
Dose
- You will start injecting Palynziq at the lowest dose. You will use the 2.5 mg syringe once a week for at least the first 4 weeks. The 2.5 mg syringe has a white plunger.
- Your doctor will gradually increase the dose or frequency of Palynziq injections and will tell you how long to inject each dose. The gradual increase in dose over time allows your body to adapt to the medicine.
- The goal is to reach a daily dose that reduces blood phenylalanine levels within the target range of 120 to 600 micromol/l and does not cause too many side effects. Normally, patients need a daily dose of 20 mg, 40 mg, or 60 mg to reach their target blood phenylalanine level.
Example of steps to reach target blood phenylalanine level
Dose of Palynziq and frequency of injection | Color of the syringe |
2.5 mg once a week | White plunger |
2.5 mg twice a week | |
10 mg once a week | Green plunger |
10 mg twice a week | |
10 mg four times a week | |
10 mg once a day | |
20 mg once a day | Blue plunger |
40 mg once a day (2 injections with the 20 mg pre-filled syringe)1 | |
60 mg once a day (3 injections with the 20 mg pre-filled syringe)1 |
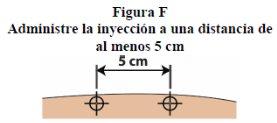
1If you need multiple injections to complete your daily dose, all injections will be administered at the same time of day, leaving a distance of at least 5 cm between each injection site. Do not divide your daily dose throughout the day.
- Your doctor will continue to check your blood phenylalanine levels during treatment and may adjust your Palynziq dose or ask you to make changes to your diet.
- Your doctor will check your blood phenylalanine levels monthly to see if the medicine is effective.
Starting Palynziq treatment
- Your healthcare professional will administer the Palynziq injection until you (or your caregiver) are able to do so.
- Your doctor will prescribe medicines that you should take before injecting Palynziq, such as paracetamol, fexofenadine, or ranitidine. These medicines help reduce the symptoms of an allergic reaction.
- A healthcare professional will observe you for at least 1 hour after you inject Palynziq to detect any signs or symptoms of a severe allergic reaction.
- Your doctor will also prescribe an adrenaline injection device to treat severe allergic reactions and will explain to you and your caregiver when and how to administer adrenaline.
- Your doctor will teach you how and when to use the adrenaline injection device. Always carry the adrenaline injection device with you.
Continuing treatment with Palynziq
- This medicine comes in pre-filled syringes with 3 different doses (2.5 mg: white plunger; 10 mg: green plunger or 20 mg: blue plunger). You may need more than one pre-filled syringe to complete the prescribed dose. Your healthcare professional will tell you which syringe, or which combination of syringes, to use and will teach you (or your caregiver) how to inject Palynziq.
- In the “Instructions for use” (section 7 of this leaflet), you will find:
- how to prepare and inject Palynziq, and
- how to properly dispose of Palynziq syringes after use.
- Your doctor will tell you how long to continue using medicines, such as paracetamol, fexofenadine, and/or ranitidine, before injecting Palynziq.
- For at least the first 6 months of treatment with Palynziq, you should be accompanied when you self-inject Palynziq and for at least 1 hour after the injection. Your companion should observe for any signs or symptoms of a severe allergic reaction and, if necessary, administer an adrenaline injection and seek urgent medical help.
- Your doctor will inform your companion of the signs and symptoms of a severe allergic reaction and how to administer an adrenaline injection.
- Your doctor will tell you if you need an observer for more than 6 months.
- Do not change your protein intake unless your doctor tells you to.
If you use more Palynziq than you should
If you use more Palynziq than you should, tell your doctor. See more details on how to proceed according to your symptoms in section 4.
If you forget to use Palynziq
If you forget a dose, inject the next dose at the usual time. Do not inject two doses of Palynziq to make up for forgotten doses.
If you stop using Palynziq
If you stop using Palynziq, your blood phenylalanine levels may increase. Talk to your doctor before stopping treatment with Palynziq.
If you have any other questions about using this medicine, ask your doctor.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Allergic reactions are very common (may affect more than 1 in 10 people) and vary in severity. The symptoms of an allergic reaction may include skin rash, itching, swelling of the head or face, itching or tearing of the eyes, coughing, and wheezing. Your doctor will tell you how to treat allergic reactions according to their severity and will prescribe other medicines to treat the reaction. Some of these allergic reactions can be more serious, as described below, and require immediate attention.
Serious side effects include:
- Sudden severe allergic reactions: (Common – may affect up to 1 in 10 people).Stop injecting Palynziq if you notice any severe sudden signs of allergy or a combination of the signs listed below:
- swelling of the face, eyes, lips, mouth, throat, tongue, hands, or feet;
- difficulty breathing or wheezing;
- a feeling of tightness in the throat or a feeling of choking;
- difficulty swallowing or speaking;
- a feeling of dizziness or fainting;
- loss of control of urine or stool;
- rapid heartbeat;
- hives (a raised, itchy rash) that spread quickly;
- redness;
- severe stomach pain, vomiting, or diarrhea.
Use the adrenaline injection device according to the doctor's instructions and seek urgent medical help.Your doctor will prescribe an adrenaline injection device to treat severe allergic reactions and will explain to you and your caregiver when and how to administer adrenaline. Always carry the adrenaline injection device with you.
Contact your doctor immediatelyif you:
- have a type of allergic reaction called serum sickness, which includes a combination of fever (high temperature), rash, muscle and joint pain (Common – may affect up to 1 in 10 people).
Other side effects
Very common: may affect more than 1 in 10 people
- redness of the skin, swelling, bruising, pain, or tenderness at the injection site;
- joint pain;
- reduction of proteins C3 and C4 of the complement factor C4 (which are parts of the immune system) in blood tests;
- allergic reaction;
- low blood phenylalanine levels in blood tests;
- headache;
- skin rash;
- stomach pain;
- feeling sick or nausea;
- vomiting;
- hives (an itchy rash that causes itching);
- itching;
- hair loss or thinning;
- coughing;
- increased C-reactive protein (CRP) in blood tests (CRP is a protein that indicates you have inflammation);
- swollen glands in the neck, armpits, or groin;
- redness of the skin;
- muscle pain.
Common: may affect up to 1 in 10 people
- joint stiffness;
- joint swelling;
- muscle stiffness;
- skin rash with small bumps;
- blistering or peeling of the outer layer of the skin.
Reporting of side effects
If you experience any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly through the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Palynziq
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the label of the syringe, the blister pack, and the carton after “EXP”. The expiry date refers to the last day of the month shown.
Store in a refrigerator (between 2°C and 8°C). Do not freeze.
If necessary, Palynziq can be stored outside of the refrigerator (below 25°C), in its sealed blister pack, for a single period of up to 30 days, away from any heat source. Write the date you removed it from the refrigerator on the blister pack. Once removed from the refrigerator, the medicine should not be put back in the refrigerator.
Do not use this medicine if the pre-filled syringe is damaged or if you notice that the solution has lost its color, is cloudy, or has particles.
Dispose of the syringes safely. Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Container Contents and Additional Information
Composition of Palynziq
- The active ingredient is pegvaliasa.
Each 2.5 mg prefilled syringe contains 2.5 mg of pegvaliasa in 0.5 ml of solution. Each 10 mg prefilled syringe contains 10 mg of pegvaliasa in 0.5 ml of solution. Each 20 mg prefilled syringe contains 20 mg of pegvaliasa in 1 ml of solution.
- The other components are tromethamine, tromethamine hydrochloride, sodium chloride (see section 2 for more information), trans-cinnamic acid, and water for injectable preparations.
Appearance of Palynziq and Container Contents
Palynziq injectable solution is a clear to slightly opalescent and colorless to pale yellow solution. The prefilled syringe includes an automatic needle shield.
2.5 mg prefilled syringe (white plunger):
Each 2.5 mg carton contains 1 prefilled syringe.
10 mg prefilled syringe (green plunger):
Each 10 mg carton contains 1 prefilled syringe.
20 mg prefilled syringe (blue plunger):
Each 20 mg carton contains 1 or 10 prefilled syringes.
Only some pack sizes may be marketed.
Marketing Authorization Holder and Manufacturer
BioMarin International Limited
Shanbally, Ringaskiddy
County Cork
Ireland
P43 R298
Date of Last Revision of this Leaflet: MM/YYYY.
Other Sources of Information
Detailed information on this medicinal product is available on the European Medicines Agency website: http://www.ema.europa.eu. There are also links to other websites on rare diseases and orphan medicines.
- Instructions for Use
BEFORE YOU START
Read these instructions for use before you start using the Palynziq prefilled syringe and each time you are prescribed something new. There may be new information. Also, consult your healthcare professional about your medical condition or treatment.
Follow these instructions carefully when using Palynziq. If your healthcare professional decides that you or your caregiver can administer the Palynziq injections at home, you will be trained on how to do it before giving the first injection. Do notinject Palynziq until your healthcare professional has taught you or your caregiver how to do it.
Consult your healthcare professional if you have any questions about how to inject Palynziq correctly.
Do not share prefilled syringes with anyone.
To see the storage instructions, refer to section 5 "Storage of Palynziq" of this leaflet.
Important Things to Know About Using the Palynziq Prefilled Syringe:
- Use each Palynziq prefilled syringe only once. Do notuse a Palynziq syringe more than once.
- Neverpull back the plunger.
- Do notremove the needle shield until you are ready to administer the injection.
The figure A below shows the appearance of the prefilled syringe before use.
Figure A
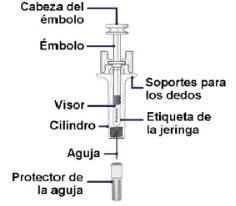
Select the Correct Palynziq Prefilled Syringes for Your Dose:
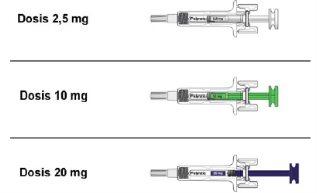
When you receive your Palynziq prefilled syringes, check that the name "Palynziq" appears on the cartons.
- Palynziq prefilled syringes come in 3 different doses: 2.5 mg, 10 mg, and 20 mg.
- You may need more than one prefilled syringe to complete the prescribed dose. Your healthcare professional will tell you which syringe, or which combination of syringes, to use. If you are unsure, consult your healthcare professional.
- Before injecting Palynziq, check each carton and syringe to make sure you have the correct prefilled syringe for the prescribed dose.
Figure B
PREPARATION FOR INJECTION
Step 1: Gather Everything You Need:
Gather everything you need for the injection and place it on a flat and clean surface. Remove the required number of cartons from the refrigerator.
This is what you will need for the Palynziq injection:
- Prefilled Palynziq syringe(s) in sealed tray(s); each tray contains 1 syringe;
- Cotton balls or swabs;
- 1 alcohol swab;
- 1 band-aid;
- 1 container for disposing of sharp objects or a puncture-resistant container.
Step 2: Remove the Palynziq Tray(s) from the Carton and Check the Expiration Date:
- Remove the required number of cartons from the refrigerator. Check the expiration date on the carton. If the expiration date has passed, do not use the prefilled syringe from that carton.
- Open each carton and remove the sealed tray you need for your dose.
- Place each sealed tray on a flat and clean surface, out of the reach of children and pets.
- Store the carton with the remaining trays in the refrigerator. If you do not have a refrigerator, see section 5 "Storage of Palynziq" of this leaflet.
Step 3: Before Opening the Palynziq Tray(s), Let it Sit at Room Temperature for 30 Minutes:
Let the sealed tray(s) sit at room temperature for at least 30 minutes. If you inject Palynziq when it is still cold, it may be uncomfortable.
- Do notheat the prefilled syringe in any other way. Do notheat it in the microwave or in hot water.
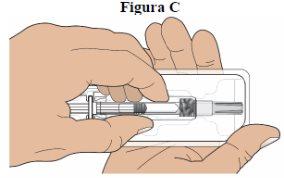
Step 4: Remove the Syringe from the Tray:Remove the tray cover. Hold the prefilled syringe by the middle of the cylinder and remove it from the tray (see figure C).
|
|
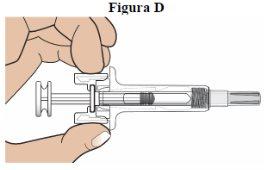
Step 5: Check the Dose on the Syringe and for Particles:
Check the syringe label to make sure you have the correct dose for the prescribed dose. Look at the liquid through the window (see figure D). The liquid should appear clear and colorless to pale yellow. It is normal to have an air bubble.
|
|
INJECTION OF PALYNZIQ
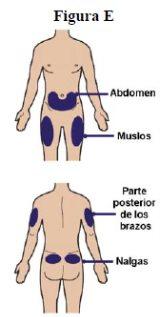
Step 6:Choose the injection site. The recommended sites for injection are:
If the caregiver gives you the injection, the upper buttocks and the upper back of the arms can also be used (see figure E). Note:
|
|
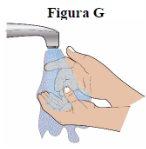
Step 7:Wash your hands well with soap and water (see figure G). |
|
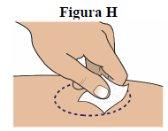
Step 8:Clean the chosen site with an alcohol swab. Let the skin air dry for at least 10 seconds before administering the injection (see figure H).
|
|
Inject Palynziq
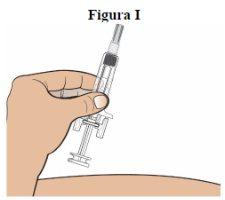
Step 9:Hold the cylinder of the prefilled syringe with one hand, with the needle facing away from your body (see figure I).
|
|
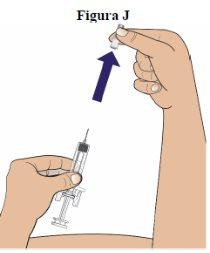
Step 10:Remove the needle shield by pulling it straight off (see figure J).
You may see a drop of liquid on the tip of the needle. This is normal. Do notremove it. Dispose of the needle shield in a puncture-resistant container. |
|
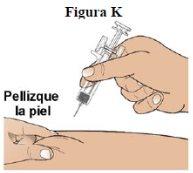
Step 11:Hold the cylinder of the prefilled syringe with one hand, between your thumb and index finger. With your other hand, pinch the skin around the injection site. Hold the skin firmly (see figure K).
|
|
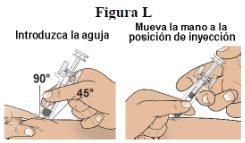
Step 12:With a quick motion, insert the needle all the way into the pinched skin at an angle of 45 to 90 degrees (see figure L). Release the skin. With that hand, hold the lower part of the syringe firmly. Place your thumb from the other hand on the plunger head (see figure L). |
|
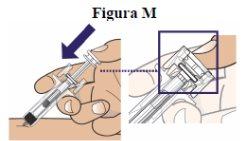
Step 13:With your thumb, push the plunger slowly but steadily until it stops moving to inject all the medicine (see figure M). For the 10 mg and 20 mg doses, you may need to apply more pressure to inject all the medicine |
|
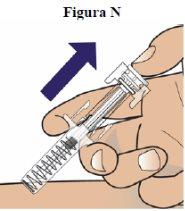
Step 14:Slowly move your thumb up to release the plunger and allow the needle to be automatically covered by the syringe cylinder (see figure N) |
|
Treat the Injection Site
Step 15:Treat the injection site (if necessary). If you see drops of blood at the injection site, press it with a cotton ball or swab for 10 seconds, approximately. You can cover the injection site with a band-aid, if necessary. | |
If More Than One Syringe is Needed: Step 16:If your healthcare professional tells you to use more than one syringe for your dose, repeat steps 4 to 15 for each syringe you use.
|
|
AFTER THE INJECTION
Dispose of Used Syringes.
Place used needles and syringes in a puncture-resistant container immediately after use. Consult your doctor, pharmacist, or nurse about the proper way to dispose of the container. Dispose of the syringes safely.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to PALYNZIQ 10 mg Injectable Solution in Pre-filled SyringeDosage form: INJECTABLE, 2.5 mgActive substance: pegvaliaseManufacturer: Biomarin International LimitedPrescription requiredDosage form: INJECTABLE, 20 mgActive substance: pegvaliaseManufacturer: Biomarin International LimitedPrescription requiredDosage form: INJECTABLE INFUSION, 100 UActive substance: laronidaseManufacturer: Sanofi B.V.Prescription required
Online doctors for PALYNZIQ 10 mg Injectable Solution in Pre-filled Syringe
Discuss questions about PALYNZIQ 10 mg Injectable Solution in Pre-filled Syringe, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions