
ORFADIN 4 mg/ml ORAL SUSPENSION

How to use ORFADIN 4 mg/ml ORAL SUSPENSION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Orfadin 2 mg hard capsules
Orfadin 5 mg hard capsules
Orfadin 10 mg hard capsules
Orfadin 20 mg hard capsules
nitisinone
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Orfadin and what is it used for
- What you need to know before you take Orfadin
- How to take Orfadin
- Possible side effects
- Storage of Orfadin
- Contents of the pack and other information
1. What is Orfadin and what is it used for
Orfadin contains the active substance nitisinone. Orfadin is used to treat:
- a rare disease called hereditary tyrosinemia type 1 in adults, adolescents, and children (of any age range).
- a rare disease called alkaptonuria (AKU) in adults
In these diseases, your body cannot fully break down the amino acid tyrosine (amino acids are the basic building blocks of proteins), forming toxic substances. These substances accumulate in your body. Orfadin blocks the breakdown of tyrosine, and the toxic substances are not formed.
For the treatment of hereditary tyrosinemia type 1, you must follow a special diet while taking this medicine, because tyrosine will still be in your body. This diet is based on a low content of tyrosine and phenylalanine (another amino acid).
For the treatment of AKU, your doctor may advise you to follow a special diet.
2. What you need to know before you take Orfadin
Do not take Orfadin
- if you are allergic to nitisinone or any of the other ingredients of this medicine (listed in section 6).
Do not breastfeed while taking this medicine (see section "Pregnancy and breastfeeding").
Warnings and precautions
Talk to your doctor or pharmacist before you start taking Orfadin.
- An ophthalmologist will examine your eyes before treatment and regularly during treatment with nitisinone. If you notice redness of the eyes or any other effect on the eyes, contact your doctor immediately for an ophthalmological examination. Eye problems (see section 4) may be a sign of inadequate diet control.
During treatment, blood samples will be taken to check if the treatment is adequate and to ensure that there are no side effects causing blood alterations.
If you receive Orfadin for the treatment of hereditary tyrosinemia type 1, you will have regular liver checks because the disease affects the liver.
Your doctor should check you every 6 months. If you experience any side effects, it is recommended to use shorter time intervals.
Other medicines and Orfadin
Tell your doctor or pharmacist if you are taking, have recently taken, or might take any other medicines.
Orfadin may interfere with the effect of other medicines, such as:
- medicines for epilepsy (such as phenytoin)
- medicines to prevent blood clot formation (such as warfarin)
Using Orfadin with food
It is recommended to take the oral suspension with food.
Pregnancy and breastfeeding
The safety of this medicine in pregnant women and breastfeeding women has not been studied.
Talk to your doctor if you plan to become pregnant. If you become pregnant, you should contact your doctor immediately.
Do not breastfeed while taking this medicine (see section "Do not take Orfadin").
Driving and using machines
The influence of this medicine on the ability to drive and use machines is small. However, if you experience side effects that affect your vision, do not drive or use machines until your vision has returned to normal (see section 4 "Possible side effects").
Orfadin contains sodium, glycerol, and sodium benzoate
This medicine contains 0.7 mg (0.03 mmol) of sodium per ml.
A dose of 20 ml of oral suspension (10 g of glycerol) or more may cause headache, stomach upset, and diarrhea.
Sodium benzoate may increase jaundice (yellow color of the skin and eyes) in premature or full-term newborns with jaundice and lead to kernicterus (brain damage caused by bilirubin deposits in the brain). In newborns, blood bilirubin levels (a substance that at high concentrations causes yellow color of the skin) should be strictly controlled. If levels are markedly higher than they should be, especially in premature babies with risk factors such as acidosis (low blood pH) or low albumin concentration (a blood protein), treatment with Orfadin capsules should be considered instead of the oral suspension until plasma bilirubin concentrations have normalized.
3. How to take Orfadin
Follow the instructions for administration of the medicine exactly as indicated by your doctor. If you are in doubt, ask your doctor or pharmacist.
Follow carefully the instructions below for preparation and administration of the dose to ensure that the correct dose is administered.
For hereditary tyrosinemia type 1, treatment with this medicine should be initiated and supervised by a doctor with experience in the treatment of the disease (hereditary tyrosinemia type 1).
For hereditary tyrosinemia type 1, the recommended daily dose is 1 mg/kg body weight administered orally. Your doctor will adjust the dose individually.
It is recommended to administer the dose once a day. However, due to limited data in patients with a body weight <20 kg, in this patient population, it is recommended to divide the total daily dose into two doses per day.< p>
For AKU, the recommended dose is 10 mg once a day.
The oral suspension should be administered directly into the mouth without dilution using the oral syringe.
Orfadin should not be injected. Do not attach a needle to the syringe.
How to prepare the dose to be administered
The dose prescribed by your doctor should be administered in ml of suspensionand not in mg. This is because the oral syringe to be used to extract the correct dose of the product from the bottle is marked in ml. If your prescription is in mg, contact your doctor or pharmacist for advice.
The pack contains a bottle of medicine with a child-resistant closure, an adapter for the bottle, and three oral syringes (1 ml, 3 ml, and 5 ml) Always use these oral syringes to take the medicine.
- The 1 ml oral syringe (the smallest) is graduated between 0.1 ml and 1 ml in small marks of 0.01 ml. It is used to measure doses of up to 1 ml.
- The 3 ml oral syringe (the medium) is graduated between 1 ml and 3 ml in small marks of 0.1 ml. It is used to measure doses of more than 1 ml and up to 3 ml.
- The 5 ml oral syringe (the largest) is graduated between 1 ml and 5 ml in small marks of 0.2 ml. It is used to measure doses of more than 3 ml.
It is important that you use the correct oral syringe to take the medicine. Your doctor, pharmacist, or nurse will indicate how to use the oral syringe based on the prescribed dose.
How to prepare a new bottle of medicine for first use:
Before taking the first dose, the bottle should be shaken vigorously, as during prolonged storage, the particles form a solid precipitate at the bottom of the bottle. Follow the instructions described below:
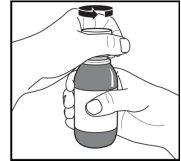
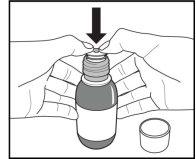
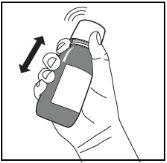
Figure A. Figure B. Figure C.
- Take the bottle out of the refrigerator and write the date on the label of the bottle.
- Shake the bottle vigorously for at least 20 secondsuntil the solid cake at the bottom of the bottle is completely dispersed (Figure A).
- Remove the child-resistant closure; to do this, press it down firmly and turn it counterclockwise (Figure B).
Place the open bottle upright on the table. Push the plastic adapter firmly into the neck of the bottle as far as it will go (Figure C) and close the bottle with the child-resistant closure.
For subsequent doses, follow the instructions below "How to prepare a dose of the medicine"
How to prepare a dose of the medicine
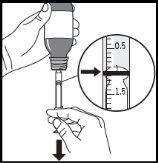
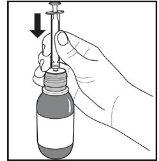
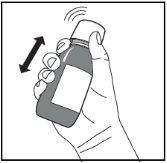
Figure D. Figure E. Figure F.
- Shake the bottle vigorously for at least 5 seconds (Figure D).
- Immediately, remove the child-resistant closure and open the bottle.
- Push the plunger of the oral syringe down to the bottom.
- Keep the bottle in a vertical position and insert the oral syringe firmly into the adapter hole at the top of the bottle (Figure E).
- Slowly turn the bottle upside down without removing the oral syringe (Figure F).
- To obtain the prescribed dose (ml), slowly pull the plunger down until the top edge of the black ring is exactly level with the line indicating the dose (Figure F). If you see any air bubbles inside the filled oral syringe, push the plunger back until the bubbles disappear. Then pull the plunger down again until the top edge of the black ring is exactly level with the line indicating the dose.
- Place the bottle back in a vertical position. Gently turn the oral syringe while pulling it out of the bottle.
- The dose should be administered immediately into the mouth (without dilution) to avoid the formation of a precipitate in the oral syringe. The oral syringe should be emptied slowlyto allow the patient to swallow the product; if the medicine comes out in a fast stream, it may cause choking.
- Immediately replace the child-resistant closure. The bottle adapter should not be removed.
- The bottle can be stored at room temperature (not above 25 °C).
Cleaning:
Clean the oral syringe immediatelywith water. Separate the plunger from the syringe cylinder and rinse both with water. Shake off excess water and let the oral syringe air dry in a disassembled state until you need to assemble it again for a new administration.
If you take more Orfadin than you should
If you have taken more of this medicine than you should, contact your doctor or pharmacist immediately.
If you forget to take Orfadin
Do not take a double dose to make up for forgotten doses. If you have forgotten to take a dose, contact your doctor or pharmacist.
If you stop taking Orfadin
If you think the effect of the medicine is too strong or too weak, talk to your doctor. Do not change the dose or stop treatment without talking to your doctor first.
If you have any further questions on the use of this product, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
If you notice any side effect related to the eyes, contact your doctor immediately for an ophthalmological examination. Treatment with nitisinone increases tyrosine levels in the blood, which may cause eye-related symptoms. In patients with hereditary tyrosinemia type 1, frequent eye side effects (may affect more than 1 in 100 people) due to higher tyrosine levels are eye inflammation (conjunctivitis), corneal opacity and inflammation (keratitis), sensitivity to light (photophobia), and eye pain. Eyelid inflammation (blepharitis) is an uncommon side effect (may affect up to 1 in 100 people).
In patients with AKU, eye irritation (keratopathy) and eye pain are very common side effects (may affect more than 1 in 10 people).
The following are other side effects reported in patients with hereditary tyrosinemia type 1:
Other common side effects
- decrease in platelet count (thrombocytopenia) and white blood cell count (leucopenia), reduction of certain types of white blood cells (granulocytopenia).
Other uncommon side effects
- increase in white blood cell count (leucocytosis),
- itching (pruritus), skin inflammation (exfoliative dermatitis), rash.
The following are other side effects reported in patients with AKU:
Other common side effects
- bronchitis,
- pneumonia,
- itching (pruritus), rash.
Reporting of side effects
If you experience any side effects, talk to your doctor, pharmacist, or nurse, even if they are not listed in this leaflet. You can also report side effects directly through the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Orfadin
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the bottle and carton after "EXP". The expiry date is the last day of the month stated.
Store in a refrigerator (between 2 °C and 8 °C). Do not freeze.
Keep the bottle in a vertical position.
After first opening, the medicine can be stored for a single period of 2 months at a temperature not above 25 °C, after which it should be discarded.
Do not forget to write the date you first opened the bottle on the bottle label.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Contents of the pack and other information
Composition of Orfadin
- The active substance is nitisinone. Each ml contains 4 mg of nitisinone.
- The other ingredients are hypromellose, glycerol (see section 2), polysorbate 80, sodium benzoate (E211) (see section 2), citric acid monohydrate, sodium citrate (see section 2), artificial strawberry flavor, and purified water.
Appearance of the product and contents of the pack
The oral suspension is a white, opaque, and slightly viscous suspension. Before shaking the bottle, it may appear as a solid cake at the bottom with a slightly opalescent supernatant.
It is presented in a 100 ml amber-colored bottle with a white child-resistant closure.
Each bottle contains 90 ml of oral suspension.
Each pack contains a bottle, a bottle adapter, and 3 oral syringes.
Marketing authorisation holder
Swedish Orphan Biovitrum International AB
SE-112 76 Stockholm
Sweden
Manufacturer
Apotek Produktion & Laboratorier AB
Celsiusgatan 43
SE-212 14 Malmö
Sweden
Apotek Produktion & Laboratorier AB
Prismavägen 2
SE-141 75 Kungens Kurva
Sweden
Date of last revision of this leaflet: 04/2024.
Detailed information on this medicine is available on the European Medicines Agency website: http://www.ema.europa.eu/. There are also links to other websites on rare diseases and orphan medicines.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to ORFADIN 4 mg/ml ORAL SUSPENSIONDosage form: CAPSULE, 10 mgActive substance: nitisinoneManufacturer: Dipharma Arzneimittel GmbhPrescription requiredDosage form: CAPSULE, 2 mgActive substance: nitisinoneManufacturer: Dipharma Arzneimittel GmbhPrescription requiredDosage form: CAPSULE, 20 mgActive substance: nitisinoneManufacturer: Dipharma Arzneimittel GmbhPrescription required
Online doctors for ORFADIN 4 mg/ml ORAL SUSPENSION
Discuss questions about ORFADIN 4 mg/ml ORAL SUSPENSION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















