OKEDI 75 mg powder and solvent for prolonged-release injectable suspension

How to use OKEDI 75 mg powder and solvent for prolonged-release injectable suspension
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
OKEDI 75 mg powder and solvent for prolonged-release injectable suspension
risperidone
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is OKEDI and what is it used for
- What you need to know before you use OKEDI
- How to use OKEDI
- Possible side effects
- Storage of OKEDI
- Contents of the pack and other information
1. What is OKEDI and what is it used for
OKEDI contains the active substance risperidone, which belongs to a group of medicines called «antipsychotics».
OKEDI is used in adult patients to treat schizophrenia, a disorder in which you may hear, see, or feel things that are not there, believe things that are not true, or feel unusually suspicious or confused.
OKEDI is indicated for patients who have shown tolerability and effectiveness to oral risperidone (e.g., tablets).
OKEDI may help to relieve the symptoms of the disease and prevent them from coming back.
2. What you need to know before you use OKEDI
Do not use OKEDI:
- If you are allergic (hypersensitive) to risperidone or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Consult your doctor or pharmacist before starting to use OKEDI if:
- You have heart problems. Examples include irregular heartbeat, if you are prone to having low blood pressure or if you are using medicines for blood pressure. OKEDI may cause low blood pressure. It may be necessary to adjust the dose.
- You know that you have any factor that would make you more likely to suffer a stroke, such as high blood pressure, cardiovascular disorder, or problems with the blood vessels in the brain.
- You have ever had involuntary movements of the tongue, mouth, and face.
- You have ever had a condition whose symptoms include high temperature, muscle stiffness, sweating, or reduced level of consciousness (also known as malignant neuroleptic syndrome).
- You have Parkinson's disease.
- You have dementia.
- You know that you have previously had low levels of white blood cells (which may have been caused by other medicines or not).
- You are diabetic.
- You have epilepsy.
- You are male and have ever had a prolonged or painful erection.
- You have problems controlling body temperature or feel excessive heat.
- You have kidney problems.
- You have liver problems.
- You have an abnormally high level of the hormone prolactin in the blood or a tumor that may depend on prolactin.
- You or a family member have a history of blood clots, as antipsychotics have been associated with the formation of blood clots.
If you are not sure if the above options apply to your case, consult your doctor or pharmacist before starting to use risperidone or OKEDI.
During treatment
Very rarely in patients taking risperidone, dangerously low levels of a type of white blood cell necessary to fight infections in the blood have been observed. Therefore, your doctor may need to check your white blood cell counts before and during treatment.
Although you have previously tolerated oral risperidone, allergic reactions have rarely occurred after receiving OKEDI injections. Seek medical attention immediately if you have a rash, swelling of the throat, itching, or breathing problems, as these may be signs of a severe allergic reaction.
OKEDI may cause weight gain. Significant weight gain can negatively affect health. Your doctor should weigh you periodically.
In patients using OKEDI, diabetes mellitus or worsening of pre-existing diabetes mellitus has been observed. Therefore, your doctor should check for signs of high blood sugar levels. In patients with pre-existing diabetes mellitus, blood glucose levels should be checked periodically.
OKEDI usually increases the levels of a hormone called «prolactin». This can cause side effects such as menstrual disorders or fertility problems in women, and breast swelling in men (see section 4, «Possible side effects»). If these side effects occur, it is recommended to evaluate the prolactin level in the blood.
During eye surgery for cataracts, problems may arise that can cause eye damage. If you plan to have eye surgery, make sure to inform your ophthalmologist that you are using this medicine.
Children and adolescents
Do not give this medicine to children and adolescents under 18 years of age.
Other medicines and OKEDI
Tell your doctor or pharmacist if you are taking, have recently taken, or might take any other medicines.
It is especially important that you talk to your doctor or pharmacist if you are taking any of the following:
- Medicines that act on the brain, for example, to calm down (benzodiazepines) or some pain medicines (opioids), for allergy (some antihistamines), as OKEDI may increase the sedative effect of all of them.
- Medicines that can change the electrical activity of the heart, such as medicines to treat malaria, heart rhythm problems, allergies (antihistamines), some antidepressants, or other medicines for mental problems.
- Medicines that slow down the heartbeat.
- Medicines that cause low potassium levels in the blood (such as certain diuretics).
- Medicines to treat high blood pressure. OKEDI may lower blood pressure.
- Medicines for Parkinson's disease (such as levodopa).
- Medicines that increase the activity of the central nervous system (psychostimulants, such as methylphenidate).
- Diuretics used to treat heart problems or swelling of parts of the body due to excess fluid accumulation (e.g., furosemide or chlorothiazide). OKEDI, alone or in combination with furosemide, may increase the risk of stroke or death in elderly people with dementia.
The following medicines may reduce the effect of risperidone:
- Rifampicin (a medicine to treat some infections).
- Carbamazepine, phenytoin (medicines for epilepsy).
- Phenobarbital.
If you start taking these medicines or stop taking them, you may need a different dose of risperidone.
The following medicines may increase the effect of risperidone:
- Quinidine (used for certain types of heart disease).
- Antidepressants (such as paroxetine, fluoxetine, tricyclic antidepressants).
- Medicines known as beta-blockers (used to treat high blood pressure).
- Phenothiazines (such as medicines used to treat psychosis or to calm down).
- Cimetidine, ranitidine (stomach acid blockers).
- Itraconazole and ketoconazole (medicines to treat fungal infections).
- Certain medicines for the treatment of HIV/AIDS, such as ritonavir.
- Verapamil, which is used to treat high blood pressure and/or heart rhythm disorders.
- Sertraline and fluvoxamine, medicines to treat depression and other psychiatric disorders.
If you start taking these medicines or stop taking them, you may need a different dose of risperidone.
If you are not sure if the above options apply to your case, consult your doctor or pharmacist before starting to use OKEDI.
Using OKEDI with food, drinks, and alcohol
You should avoid drinking alcohol when using OKEDI.
Pregnancy, breastfeeding, and fertility
- If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine. Your doctor will decide whether you can use it.
- The following symptoms may appear in newborns of mothers who have used risperidone during the last trimester (last three months of pregnancy): shaking, muscle stiffness and/or weakness, drowsiness, agitation, breathing problems, and difficulty feeding. If your baby has any of these symptoms, you may need to consult your doctor.
- OKEDI may increase the levels of a hormone called «prolactin» that can affect fertility (see section 4, «Possible side effects»).
Driving and using machines
During treatment with OKEDI, dizziness, tiredness, and vision problems may occur. Do not drive or use any tools or machines without first consulting your doctor.
3. How to use OKEDI
A healthcare professional will administer OKEDI to you by intramuscular injection in the arm or buttock every 28 days. The injections should be alternated between the right and left sides.
The recommended dose is 75 mg every 28 days, but a higher dose of 100 mg every 28 days may be necessary. Your doctor will decide which dose of OKEDI is right for you.
If you are currently being treated with other antipsychotics different from risperidone, but have previously used risperidone, you should start taking oral risperidone at least 6 days before starting treatment with OKEDI.
If you have never taken any form of risperidone, you should start taking oral risperidone at least 14 days before starting treatment with OKEDI. Your doctor will determine the duration of the oral risperidone administration period.
If you have kidney problems
The use of OKEDI is not recommended in patients with moderate to severe renal impairment.
If you use more OKEDI than you should
- Consult a doctor immediately.
- In case of overdose, you may feel drowsy or tired, have abnormal body movements, problems standing and walking, dizziness due to low blood pressure, or abnormal heartbeats or seizures.
If you stop using OKEDI
You will lose the effects of the medicine. Do not stop using this medicine unless your doctor tells you to, as the symptoms may come back.
It is important that you do not miss the appointments you have every 28 days when you are supposed to receive the injections of this medicine. If you cannot attend an appointment, make sure to inform your doctor immediately to schedule another date when you can go to receive the injection.
If you have any other questions about the use of this medicine, ask your doctor or pharmacist.
4. Possible Adverse Effects
Like all medicines, this medicine can cause adverse effects, although not all people suffer from them.
Consult a doctor or go immediately to the nearest emergency service if you experience the following rare adverse effect (may affect up to 1 in 100 people):
- You have tardive dyskinesia (involuntary movements or tics in the face, tongue, or other parts of the body that you cannot control).
Consult a doctor or go immediately to the nearest emergency service if you experience any of the following rare adverse effects (may affect up to 1 in 1000 people):
- You have blood clots in the veins, especially in the legs (symptoms include swelling, pain, and redness of the legs), which can move through the blood vessels to the lungs and cause chest pain and difficulty breathing.
- You have fever, muscle stiffness, sweating, or reduced level of consciousness (a disorder known as "neuroleptic malignant syndrome").
- You are male and have a prolonged or painful erection, which is known as priapism.
- You have a severe allergic reaction characterized by fever; swelling of the mouth, face, lip, or tongue; difficulty breathing; itching; skin rash; or low blood pressure (anaphylactic or angioedema reaction). Although you have previously tolerated oral risperidone, allergic reactions can rarely occur after receiving OKEDI injections.
- Your urine is dark red or brown, or you have significantly reduced urine output along with muscle weakness or difficulty moving your arms and legs. These may be signs of rhabdomyolysis (rapid muscle damage).
- You have weakness or dizziness, fever, chills, or sores in the mouth. These may be signs of a very low white blood cell count (a type of white blood cell that helps fight infections).
These other adverse effects may also occur:
Very common adverse effects (may affect more than 1 in 10 people):
- Difficulty falling asleep or staying asleep.
- Parkinsonism: movement disorders that can include slow or impaired movements, feeling of stiffness or muscle tension, and sometimes even a feeling that movements "freeze" and then you can move again. Other signs include slow, shuffling gait, resting tremor, increased salivation or drooling, and loss of facial expression.
- Headache.
Common adverse effects (may affect up to 1 in 10 people):
- Pneumonia (lung infection), bronchitis (infection of the main airways of the lungs), sinus infection, urinary tract infection, ear infection, flu, flu-like symptoms, sore throat, cough, nasal congestion, fever, eye infection, or conjunctivitis.
- Increased levels of a hormone called "prolactin" that is detected in a blood test. Symptoms of high prolactin levels are rare and in males may include breast swelling, difficulty having or maintaining erections, and decreased sexual desire. In females, they may include milk secretion from the breasts, menstrual disorders, absence of menstrual periods, absence of ovulation, fertility problems.
- Weight gain, increased or decreased appetite.
- Sleep disorder, irritability, depression, anxiety, feeling drowsy or being less alert.
- Dystonia (involuntary muscle contraction that causes repetitive, slow movements or abnormal postures), dyskinesia (another disorder that affects involuntary muscle movements, including repetitive, spastic, or twisting movements, or tics).
- Tremor (shaking), muscle spasms, bone or muscle pain, back pain, joint pain, falls.
- Blurred vision.
- Urinary incontinence (involuntary loss of urine).
- Fast heart rate, high blood pressure, shortness of breath.
- Abdominal pain, abdominal discomfort, vomiting, nausea, dizziness, constipation, diarrhea, indigestion, dry mouth, toothache.
- Rash, skin redness, reaction at the injection site (including discomfort, pain, redness, or swelling), swelling of the body, arms, or legs, chest pain, lack of energy and strength, fatigue, pain.
Uncommon adverse effects (may affect up to 1 in 100 people):
- Bladder infection, tonsillitis, fungal infection of the nails, infection of the deeper layers of the skin, viral infection, skin inflammation caused by mites.
- Decreased or increased white blood cell count in the blood, decreased platelet count (blood cells that help stop bleeding), anemia or decreased hematocrit (reduction of red blood cells), elevated creatine phosphokinase in the blood, elevated liver enzymes in the blood.
- Low blood pressure, drop in blood pressure when standing up, skin redness, cerebral ischemia (insufficient blood flow to the brain).
- Diabetes, high blood sugar, excessive water drinking, high cholesterol in the blood, weight loss, anorexia, high triglycerides (fat) in the blood.
- Mania (euphoric mood), confusion, decreased libido, nervousness, nightmares.
- Fainting, convulsion (epileptic seizures), feeling that everything is spinning (vertigo), ringing in the ears, ear pain.
- Overwhelming urge to move parts of the body, balance disorder, abnormal coordination, attention deficit, speech problems, loss or alteration of taste, reduced skin sensation to pain and touch, sensation of tingling, pinching, or numbness of the skin.
- Irregular and often rapid heart rate, slow heart rate, abnormal electrocardiogram (test that measures the electrical activity of heartbeats), palpitations (feeling of fluttering or pulsations in the chest), interruption of conduction between the upper and lower parts of the heart.
- Respiratory congestion, wheezing (harsh, whistling sound during breathing), nasal bleeding.
- Abnormal posture, joint stiffness, joint swelling, muscle weakness, neck pain, abnormal gait, thirst, feeling unwell, chest discomfort or general discomfort, feeling dejected.
- Stomach or intestinal irritation or infection, fecal incontinence, difficulty swallowing, excess gas or flatulence, frequent urination, inability to urinate, pain when urinating.
- Absence of menstrual periods or other problems with the menstrual cycle, milk secretion from the breasts, sexual dysfunction, breast pain or discomfort, vaginal discharge, erectile dysfunction, ejaculation disorder, breast development in males.
- Hives, skin thickening, skin disorder, intense skin itching, hair loss, eczema (areas of skin that become inflamed, itchy, cracked, and rough), dry skin, skin color change, acne, seborrheic dermatitis (red, scaly, greasy, itchy, and inflamed skin), skin injury.
- Increased sensitivity of the eyes to light, dry eyes, increased tearing.
- Allergic reaction, chills.
Rare adverse effects (may affect up to 1 in 1000 people):
- Infection.
- Inadequate secretion of the hormone that controls urine volume, dangerously excessive water drinking, excessive sugar in the urine, low blood sugar, high insulin (hormone that controls sugar levels in the blood) in the blood.
- Lack of response to stimulation, catatonia (not moving or responding while being awake), decreased level of consciousness, sleepwalking, sleep-related eating disorder, difficulty breathing during sleep (sleep apnea), rapid, shallow breathing, lung infection caused by inhaling food into the airways, pulmonary congestion, respiratory disorder, voice disorder, crackling lung sounds, lack of emotion, inability to reach orgasm.
- Problems with the blood vessels in the brain, diabetic coma, involuntary head tremor.
- Glaucoma (increased intraocular pressure), problems with eye movement, eye rotation, crust/inflammation on the eyelid margin, eye problems during cataract surgery.
- Pancreatitis, intestinal obstruction.
- Swollen tongue, cracked lips, dandruff, jaundice (yellow color of the skin and eyes), skin hardening.
- Increased breast size, breast congestion (hard, swollen, and painful breasts due to excessive milk production).
- Low body temperature, feeling cold in arms and legs.
- Drug withdrawal symptoms (also in newborns).
Very rare adverse effects (may affect up to 1 in 10,000 people):
- Potentially fatal complications of uncontrolled diabetes.
- Absence of intestinal muscle movement that causes obstruction.
Frequency not known: cannot be estimated from the available data
- Severe or potentially fatal rash with blisters and skin peeling that can start around the mouth, nose, eyes, genitals, and spread to other areas of the body (Stevens-Johnson syndrome or toxic epidermal necrolysis).
Reporting of adverse effects
If you experience any adverse effect, consult your doctor or pharmacist, even if it is a possible adverse effect that is not listed in this leaflet. You can also report them directly through the national reporting system included in Appendix V. By reporting adverse effects, you can help provide more information on the safety of this medicine.
5. Storage of OKEDI
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiration date that appears on the box, aluminum sachets, or syringe labels after (EXP). The expiration date is the last day of the month indicated.
Store below 30 °C. Store in the original packaging to protect it from moisture.
Use OKEDI immediately after reconstitution.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of the packaging and medicines that are no longer needed. This will help protect the environment.
6. Container Contents and Additional Information
OKEDI Composition
The active ingredient is risperidone.
Only the powder syringe contains the active ingredient. Once reconstituted, the administered amount of risperidone is 75 mg.
The other components are:
Prefilled powder syringe: poly(D,L-lactide-co-glycolide).
Prefilled solvent syringe: dimethyl sulfoxide.
Product Appearance and Container Contents
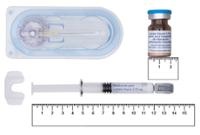
Each box of the OKEDI powder and solvent kit for prolonged-release injectable suspension contains:
- A aluminum pouch with a prefilled syringe containing powder (within the powder is the active ingredient, risperidone) and a silica gel desiccant packet. It is a loose white to off-white powder.
- A aluminum pouch with a prefilled syringe containing the solvent and a silica gel desiccant packet. The prefilled solvent syringe contains a clear solution and has red locking wings.
- A 2-inch (0.90 × 51 mm [20G]) sterile needle for intramuscular injection with a safety protector for administration in the gluteus.
- A 1-inch (0.80 × 25 mm [21G]) sterile needle for intramuscular injection with a safety protector for administration in the deltoid muscles.
Marketing Authorization Holder and Manufacturer
Laboratorios Farmacéuticos Rovi, S.A.
Julián Camarillo, 35
28037 Madrid
Spain
You can request more information about this medication by contacting the local representative of the marketing authorization holder:
Belgium Laboratorios Farmacéuticos Rovi, S.A. Julián Camarillo, 35 28037 Madrid Spain Tel: +34 91 375 62 30 | Lithuania Laboratorios Farmacéuticos Rovi, S.A. Julián Camarillo, 35 28037 Madrid Spain Tel: +34 91 375 62 30 |
Greece Laboratorios Farmacéuticos Rovi, S.A. Julián Camarillo, 35 28037 Madrid Spain Tel: +34 91 375 62 30 | Luxembourg Laboratorios Farmacéuticos Rovi, S.A. Julián Camarillo, 35 28037 Madrid Spain Tel: +34 91 375 62 30 |
Czech Republic Laboratorios Farmacéuticos Rovi, S.A. Julián Camarillo, 35 28037 Madrid Spain Tel: +34 91 375 62 30 | Hungary Laboratorios Farmacéuticos Rovi, S.A. Julián Camarillo, 35 28037 Madrid Spain Tel: +34 91 375 62 30 |
Denmark Laboratorios Farmacéuticos Rovi, S.A. Julián Camarillo, 35 28037 Madrid Spain Tel: +34 91 375 62 30 | Malta Laboratorios Farmacéuticos Rovi, S.A. Julián Camarillo, 35 28037 Madrid Spain Tel: +34 91 375 62 30 |
Germany Rovi GmbH Rudolf-Diesel-Ring 6 83607 Holzkirchen Tel: +49 8024 4782955 | Netherlands Laboratorios Farmacéuticos Rovi, S.A. Julián Camarillo, 35 28037 Madrid Spain Tel: +34 91 375 62 30 |
Estonia Laboratorios Farmacéuticos Rovi, S.A. Julián Camarillo, 35 28037 Madrid Spain Tel: +34 91 375 62 30 | Norway Laboratorios Farmacéuticos Rovi, S.A. Julián Camarillo, 35 28037 Madrid Spain Tel: +34 91 375 62 30 |
Greece BIANEΞ Α.Ε. Οδ?ς Βαρυμπ?μπης 8, 14671 Ν. Ερυθρα?α, Κηφισι? Tel. 210 8009111 | Austria Rovi GmbH Rudolf-Diesel-Ring 6 83607 Holzkirchen Germany Tel: +43 664 1340471 |
Spain Laboratorios Farmacéuticos Rovi, S.A. Julián Camarillo, 35 28037 Madrid Tel: +34 91 375 62 30 | Poland Laboratorios Farmacéuticos Rovi, S.A. Julián Camarillo, 35 28037 Madrid Spain Tel: +34 91 375 62 30 |
France ROVI 24, Rue Du Drac 38180 Seyssins Tel: +33 (0)4 76 968 969 | Portugal Laboratorios Farmacéuticos Rovi, S.A. Julián Camarillo, 35 28037 Madrid Spain Tel: +34 91 375 62 30 |
Croatia Laboratorios Farmacéuticos Rovi, S.A. Julián Camarillo, 35 28037 Madrid Spain Tel: +34 91 375 62 30 Ireland Laboratorios Farmacéuticos Rovi, S.A. Julián Camarillo, 35 28037 Madrid Spain Tel: +34 91 375 62 30 | Romania Laboratorios Farmacéuticos Rovi, S.A. Julián Camarillo, 35 28037 Madrid Spain Tel: +34 91 375 62 30 Slovenia Laboratorios Farmacéuticos Rovi, S.A. Julián Camarillo, 35 28037 Madrid Spain Tel: +34 91 375 62 30 |
Iceland Laboratorios Farmacéuticos Rovi, S.A. Julián Camarillo, 35 28037 Madrid Spain Tel: +34 91 375 62 30 | Slovak Republic Laboratorios Farmacéuticos Rovi, S.A. Julián Camarillo, 35 28037 Madrid Spain Tel: +34 91 375 62 30 |
Spain Laboratorios Farmacéuticos Rovi, S.A. Julián Camarillo, 35 28037 Madrid Tel: +34 91 375 62 30 | Poland Laboratorios Farmacéuticos Rovi, S.A. Julián Camarillo, 35 28037 Madrid Spain Tel: +34 91 375 62 30 |
Italy Rovi Biotech, S.R.L. Viale Achille Papa, 30 20149 Milano Tel: +39 02 366 877 10 | Finland Laboratorios Farmacéuticos Rovi, S.A. Julián Camarillo, 35 28037 Madrid Spain Tel: +34 91 375 62 30 |
Cyprus Laboratorios Farmacéuticos Rovi, S.A. Julián Camarillo, 35 28037 Madrid Spain Tel: +34 91 375 62 30 | Sweden Laboratorios Farmacéuticos Rovi, S.A. Julián Camarillo, 35 28037 Madrid Spain Tel: +34 91 375 62 30 |
Latvia Laboratorios Farmacéuticos Rovi, S.A. Julián Camarillo, 35 28037 Madrid Spain Tel: +34 91 375 62 30 | United Kingdom (Northern Ireland) Rovi Biotech Limited Davis House 4th Floor Suite 425 Robert Street Croydon CR0 1QQ - UK Tel: + 44 (0) 203 642 06 77 |
Date of the last revision of this prospectus: May 20, 2023.
This information is intended only for healthcare professionals.
INSTRUCTIONS FOR HEALTHCARE PROFESSIONALS
OKEDI 75 mg powder and solvent for prolonged-release injectable suspension
Important Information
To ensure the correct administration of OKEDI, it is necessary to pay close attention to these step-by-step instructions.
Use the provided components
The components included in the kit box are specifically designed to be used with OKEDI. OKEDI should only be reconstituted with the solvent supplied in the kit box.
Do not substituteANY component of the kit box.
Administer the dose immediately after reconstitution. For intramuscular use only after reconstitution.
Correct Administration
All the contents of the reconstituted syringe should be administered to ensure the administration of the indicated OKEDI dose.
Single-Use Device
- CHECK THE CONTENTS
On a clean surface, open the packets and discard the desiccant.
The OKEDI kit box contains the following:
- A aluminum pouch with a prefilled syringe of OKEDI, with a white plunger rod and white locking wings. The syringe is marked with.
- A aluminum pouch with SOLVENT for the prefilled syringe of OKEDI, with a transparent plunger rod and red locking wings. The syringe is marked with.
- Two needles for administration (21G, 1 inch for deltoid muscles [green closure cap] and 20G, 2 inches for gluteus [yellow closure cap]).
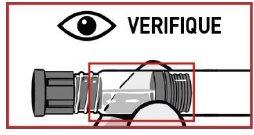
Discard the kit if any of the components are damaged.
In case you observe any foreign particles and/or variation in physical appearance, do not administer OKEDI.
1.1 Inspect the solvent syringe
MAKE SURE the contents of the solvent syringe flow normally like a liquid.
The solvent freezes below 19°C.
If it is frozen or partially frozen, before continuing, allow it to thaw by putting it in contact with your hands or leaving it at room temperature until it flows like a liquid again.
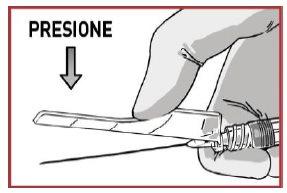
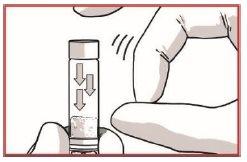
1.2 Loosen the powder from the syringe
TAP the OKEDI syringe to loosen any possible powder plug near the closure cap.
- CONNECT THE SYRINGES
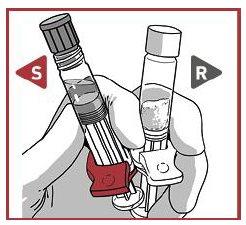
2.1 Remove the closure caps from the syringes in a vertical position
Hold both syringes in a vertical position to avoid product loss.
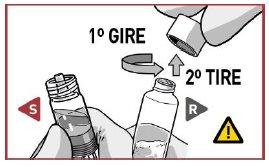
PULL the closure cap to remove it from the solvent syringe.
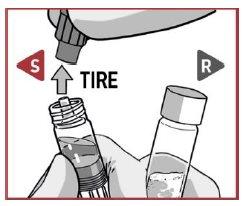
TURN the closure cap and PULL it to remove it from the powder syringe.
2.2 Connect the syringes
Place the solvent syringe S with the red locking wings OVERthe powder syringe R, or slightly tilt it when connecting them.
SCREW the syringes together until you feel a slight resistance.
MAKE SURE the powder syringe R is in a vertical position to avoid product loss.
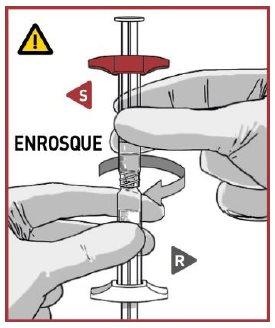
- MIX THE CONTENTS
STOP AND READ THIS SECTION BEFORE STARTING OR YOU MAY RECONSTITUTE THE MEDICATION INCORRECTLY.
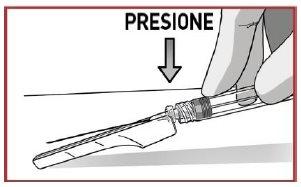
- PRESSthe solvent contents vigorously into the powder syringe.
- DO NOT WAITfor the powder to become moist and start mixing the contents QUICKLY, pressing the plungers in a FASTand alternating manner about 100 times (2 times in 1 second, approximately 1 minute).
- MAKE SUREthe medication passes from one syringe to the other to mix correctly: the medication is viscous and you will need to apply force when pressing the plunger rods.
Mixby pressing at least 100 timesin an alternating manner
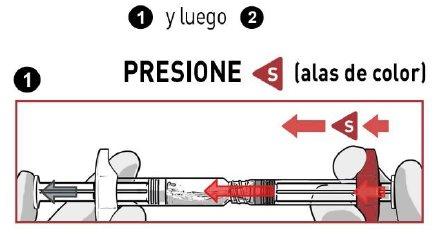
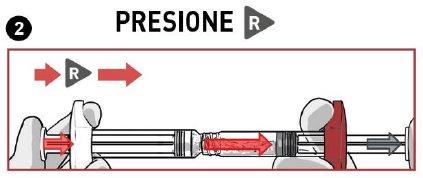

MAKE SURE the medication passes from one syringe to the other
When the medication is mixed correctly, the appearance will be a uniform suspension of white to yellowish color and thick consistency.

Once reconstituted, prepare the injection syringe immediately for administration to avoid loss of homogeneity.
- PREPARE THE INJECTION SYRINGE
4.1 Transfer the medication
Apply downward pressure on plunger rod Rand transfer all the contents to syringe Swith the colored locking wings.
MAKE SURE all the contents have been transferred.
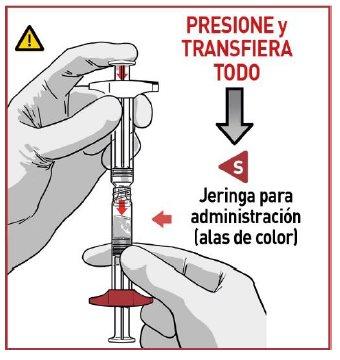
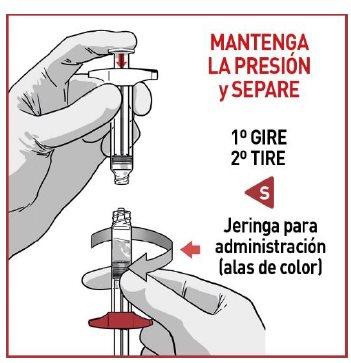
4.2 Separate the syringes
Once all the medication has been transferred, separate both syringes by unscrewing them.
OKEDI should be administered immediately to avoid loss of homogeneity.
4.3 Place the sterile needle with the safety protector
Choose the correct needle:
- Deltoid: 21G, 1 inch for the deltoid muscle (green closure cap).
- Gluteus: 20G, 2 inches for the gluteus (yellow closure cap).
Place it by turning it clockwise. Do not overtighten.
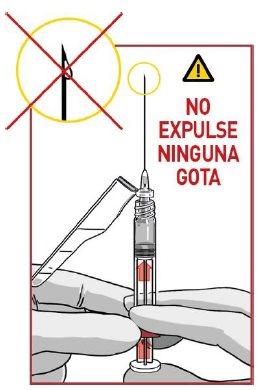
4.4 Remove excess air
Remove the needle protector and remove excess air (only large bubbles) from the syringe cylinder.
DO NOT expel any medication droplets
If you observe medication at the tip of the needle, pull the plunger slightly back to avoid spilling medication.
- ADMINISTER AND DISCARD
5.1 Inject the medication
Insert the needle fully into the muscle. DO NOT ADMINISTER THE INJECTION BY ANY OTHER ROUTE.
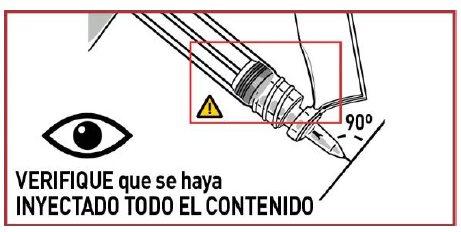
THICK MEDICATION, INJECT SLOWLY AND CONSTANTLY. MAKE SURE TO INJECT ALL OF IT.
- The injection time is longer than usual due to the viscosity of the medication.
- Wait a few seconds before removing the needle.
- Avoid accidental injection into a blood vessel.
5.2 Discard the medication
Cover the needle by applying pressure on the needle protector with your finger or a flat surface, and discard it immediately in a safe sharps container.
|
|
- Country of registration
- Average pharmacy price539.57 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to OKEDI 75 mg powder and solvent for prolonged-release injectable suspensionDosage form: TABLET, 1 mgActive substance: risperidoneManufacturer: Neuraxpharm Spain S.L.Prescription requiredDosage form: TABLET, 3 mgActive substance: risperidoneManufacturer: Neuraxpharm Spain S.L.Prescription requiredDosage form: TABLET, 6 mgActive substance: risperidoneManufacturer: Neuraxpharm Spain S.L.Prescription required
Online doctors for OKEDI 75 mg powder and solvent for prolonged-release injectable suspension
Discuss questions about OKEDI 75 mg powder and solvent for prolonged-release injectable suspension, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions