O-bi-zur 500 units powder and solvent for injectable solution


How to use O-bi-zur 500 units powder and solvent for injectable solution
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
OBIZUR500U powder and solvent for solution for injection
Susoctocog alfa
This medicinal product is subject to additional monitoring, which will allow for quick identification of new safety information. You can help by reporting any side effects you may get. The last section of this leaflet includes information on how to report side effects.
Read all of this leaflet carefully before you start using this medicine, because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is OBIZUR and what is it used for
- What you need to know before you use OBIZUR
- How to use OBIZUR
- Possible side effects
- Storage of OBIZUR
- Contents of the pack and further information
1. What is OBIZUR and what is it used for
OBIZUR contains the active substance susoctocog alfa, porcine sequence antihaemophilic factor VIII. Factor VIII is necessary for blood to clot and to stop bleeding.
In patients with acquired haemophilia, FVIII does not work properly because the patient has produced antibodies against their own factor VIII that neutralize this blood clotting factor.
OBIZUR is used for the treatment of bleeding episodes in adults with acquired haemophilia (a bleeding disorder caused by the lack of factor VIII activity due to the production of antibodies). The neutralizing effect of these antibodies against OBIZUR is lower than against human factor VIII.
OBIZUR restores the missing factor VIII activity and helps the blood to form clots at the site of bleeding.
2. What you need to know before you use OBIZUR
The product can only be administered to hospitalised patients, as clinical monitoring of the patient's bleeding condition is necessary.
Do not use OBIZUR:
- if you are allergic to susoctocog alfa or any of the other ingredients of this medicine (listed in section 6)
- if you are allergic to hamster proteins (OBIZUR may contain minimal amounts derived from the manufacturing process)
In case of doubt, consult your doctor before using this medicine.
Warnings and precautions
Consult your doctor before using OBIZUR.
There is a small possibility that you may experience an allergic reaction to OBIZUR. You should be aware of the early signs of allergic reactions (see section 4 for signs and symptoms). If any of these symptoms occur, the injection should be stopped. Severe symptoms, such as difficulty breathing and (pre-) syncope, require urgent treatment.
Patients who produce inhibitory antibodies against OBIZUR
Your doctor may check if you have inhibitory antibodies against porcine factor VIII.
Your doctor will check the factor VIII in your blood to confirm that you are receiving sufficient factor VIII. Your doctor will also check if the bleeding has stopped satisfactorily.
Tell your doctor if you have had cardiovascular disease in the past or if you have a known risk of thrombosis (diseases caused by blood clots in normal blood vessels), as it cannot be ruled out that you may experience thromboembolic diseases with the administration of high and prolonged concentrations of factor VIII.
Name and batch number
We strongly recommend that the medical professional records the name and batch number of the medicine each time OBIZUR is used, in order to maintain a link between your treatment and the batch of the medicine.
Children and adolescents
OBIZUR is not currently authorized for the treatment of patients under 18 years of age, in whom acquired haemophilia is rare.
Using OBIZUR with other medicines
Tell your doctor if you are using, have recently used or might use any other medicines. No interactions between OBIZUR and other medicines are known.
Pregnancy and breast-feeding
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor for advice before using this medicine.
Driving and using machines
OBIZUR does not affect the ability to drive and use machines.
OBIZUR contains sodium
This medicine contains 4.4 mg of sodium per millilitre once prepared.
Ask your doctor if you are on a low sodium diet.
3. How to use OBIZUR
Treatment with OBIZUR will be carried out by a doctor with experience in the care of patients with haemophilia (bleeding disorders).
Your doctor will calculate the dose of OBIZUR (in units or U) depending on your condition and body weight. The frequency and duration of administration will depend on the degree of effectiveness that OBIZUR has in your case. Normally, the substitute treatment with OBIZUR is temporary until the bleeding stops or the antibodies against your own factor VIII are eradicated.
The recommended initial dose is 200 U per kilogram of body weight administered by intravenous injection.
Your doctor will check your factor VIII activity from time to time to decide on the next dose and frequency of OBIZUR.
Bleeding usually stops within the first 24 hours; your doctor will adjust the dose and duration of OBIZUR until the bleeding stops.
The total volume of reconstituted OBIZUR should be administered at a rate of between 1 and 2 ml per minute.
Follow exactly the administration instructions of this medicine given by your doctor. If in doubt, consult your doctor again.
If you use more OBIZUR than you should
Follow exactly the administration instructions of OBIZUR given by your doctor. If you use more OBIZUR than recommended, inform your doctor as soon as possible.
If you forget to use OBIZUR
Do not use a double dose to make up for forgotten doses. Consult your doctor if you have forgotten a dose and do not know how to make up for it.
If you stop using OBIZUR
Do not stop using OBIZUR without consulting your doctor.
If you have any further questions on the use of this medicine, ask your doctor.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
If severe and sudden allergic reactions occur, the injection should be stopped immediately. Contact your doctor immediately if you have any of the following initial symptoms:
- Swelling of lips and tongue
- Stinging and pricking at the injection site
- Chills, flushing
- Hives, generalised itching
- Headache, low blood pressure
- Drowsiness (lethargy), feeling sick, restlessness
- Fast heartbeat, chest tightness
- Tingling, vomiting
- Wheezing (a high-pitched whistling sound when you breathe)
Common side effects (may affect up to 1 in 10 people)
- Production of antibodies against the medicine
Reporting of side effects
If you experience any side effects, talk to your doctor, even if they are not listed in this leaflet. You can also report side effects directly via the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of OBIZUR
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the carton, vial and pre-filled syringe after EXP. The expiry date is the last day of the month shown.
Store in a refrigerator (between 2°C and 8°C).
Do not freeze.
Use the reconstituted solution immediately and no later than 3 hours after the powder has been completely dissolved.
After reconstitution, the solution should be clear and colourless.
Do not administer it if you detect particles or a change in colour.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Marketing Authorization Holder and Manufacturer
Composition of OBIZUR
- The active substance is susoctocog alfa (antihemophilic factor VIII, porcine sequence, produced by recombinant DNA technology). Each vial of powder contains 500 U of susoctocog alfa.
- The other components of the powder are polysorbate 80, sodium chloride (see also section 2), calcium chloride dihydrate, sucrose, Tris base, Tris HCl, trisodium citrate dihydrate.
- The solvent is 1 ml of sterile water for injectable preparations.
Appearance and Packaging of the Product
A pack contains 1, 5, or 10 units of the following:
- glass vial with 500 U of OBIZUR in the form of white friable powder, provided with a rubber stopper and a flip-off type closure cap
- glass syringe preloaded with 1 ml of sterile water for injectable preparations, provided with a butyl rubber protector and a Luer connector adapter
- liquid transfer device with integrated plastic spike
Marketing Authorization Holder and Manufacturer
Marketing Authorization Holder
Baxalta Innovations GmbH
Industriestrasse 67
A-1221 Vienna
Austria
Manufacturer
Baxter AG
Industriestrasse 67
A-1221 Vienna
Austria
You can request more information about this medicinal product by contacting the local representative of the marketing authorization holder:
Belgium Baxalta Belgium SPRL Tel.: +32 2 892 62 00 | Lithuania UAB Baxter Lithuania Tel: +370 5 269 16 90 / +370 5 252 71 00 |
Bulgaria Baxalta Bulgaria EOOD Tel.: +359 2 9808482 | Luxembourg Baxalta Belgium SPRL Tel: +32 2 892 62 00 |
Czech Republic Baxter Czech spol.s r.o. Tel.: +420 225774111 | Hungary Baxter Hungary Kft Tel.: +36 1 202 1980 |
Denmark Baxalta Denmark A/S Tlf.: +45 32 70 12 00 | Malta Baxalta UK Limited Tel.: +44 1 635 798 777 |
Germany Baxalta Deutschland GmbH Tel.: +49 89 262077-011 | Netherlands Baxalta Netherlands B.V. Tel.: +31 30 799 27 77 |
Estonia OÜ Baxter Estonia Tel.: +372 6 515 120 | Norway Baxalta Norway AS Tlf.: +47 22 585 000 |
Greece Baxter Hellas ΕΠΕ Τηλ.: +30 210 28 80 000 | Austria Baxalta Österreich GmbH Tel.: +43 1 20100-0 |
Spain Baxalta Spain S.L. Tel.: +34 91 790 42 22 | Poland Baxter Polska Sp. z.o.o. Tel.: +48 22 4883 777 |
France Baxalta France SAS Tél.: +33 1 70 96 06 00 | Portugal Baxalta Portugal, Unipessoal, Lda. Tel.: +351 21 122 03 00 |
Croatia Baxter d.o.o. Tel.: +386 1 420 16 80 | Romania FARMACEUTICA REMEDIA SA Tel.: +40 21 321 16 40 |
Ireland Baxalta UK Limited Tel.: +44 1 635 798 777 | Slovenia Baxter d.o.o. Tel.: +386 1 420 16 80 |
Iceland Lyfjaver ehf. Sími: +354 533 6100 | Slovakia Baxter Slovakia, s.r.o. Tel: +421 2 3210 1150 |
Italy Baxalta Italy S.r.l. Tel.: +39 06 45224 600 | Finland Baxalta Finland Oy Puh/Tel.: +358 201 478 200 |
Cyprus Baxter Hellas ΕΠΕ Τηλ.: +30 210 28 80 000 | Sweden Baxalta Sweden AB Tel.: +46 8 50 53 26 00 |
Latvia SIA Baxter Latvia Tel.: +371 67 784 784 | United Kingdom Baxalta UK Limited Tel.: +44 1 635 798 777 |
Date of Last Revision of this Leaflet:
This medicinal product has been authorized under exceptional circumstances. This means that due to the rarity of the disease, it has not been possible to obtain complete information on this medicinal product.
The European Medicines Agency will review any new information on this medicinal product that may become available annually, and this leaflet will be updated as necessary.
Other Sources of Information
Detailed information on this medicinal product is available on the European Medicines Agency website: http://www.ema.europa.eu, and on the website of the Spanish Agency for Medicines and Health Products (AEMPS) (http://www.aemps.gob.es/). There are also links to other websites on rare diseases and orphan medicines.
This information is intended only for healthcare professionals:
INSTRUCTIONS FOR PREPARATION AND ADMINISTRATION
Preparation
Before starting reconstitution, you will need the following:
- Calculated number of powder vials
- Same number of 1 ml solvent syringes and sterile vial adapters
- Alcohol-impregnated swabs
- A large sterile syringe for introducing the final volume of the reconstituted medicinal product
The following procedures are general guidelines for the preparation and reconstitution of OBIZUR. Repeat the following reconstitution instructions for each powder vial to be reconstituted.
Reconstitution
Use an aseptic technique during the reconstitution procedure.
- Allow the OBIZUR powder vial and the preloaded solvent syringe to reach room temperature.
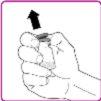
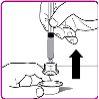
- Remove the plastic cap from the OBIZUR powder vial (Figure A).
- Rub the rubber stopper with an alcohol-impregnated swab (not included) and wait for it to dry.
- Remove the protector from the vial adapter packaging (Figure B). Do not touch the Luer connector in the center of the vial adapter. Do not remove the vial adapter from the packaging.
- Place the packaging with the vial adapter on a clean surface with the Luer connector facing up.
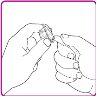
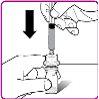
- Break the tamper-evident seal on the preloaded solvent syringe (Figure C).
- Hold the packaging with the vial adapter firmly and connect the preloaded solvent syringe to the vial adapter by pressing the syringe cone against the Luer connector in the center of the vial adapter and turning it clockwise until the syringe is securely attached. Do not overtighten (Figure D).
- Remove the plastic packaging (Figure E).
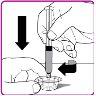
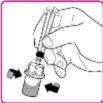
- Place the OBIZUR powder vial on a clean, flat, and hard surface. Place the vial adapter over the OBIZUR powder vial and push the filter spike of the vial adapter firmly through the rubber circle of the OBIZUR powder vial until the transparent plastic protector clicks into place on the vial (Figure F).
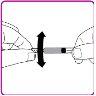
- Slowly push the plunger to inject all the solvent from the syringe into the OBIZUR powder vial.
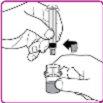
- Gently move (with a circular motion) the OBIZUR powder vial without removing the syringe until all the powder is dissolved/reconstituted (Figure G). Before administering the reconstituted solution, it must be visually inspected for particulate matter or color change. Do not use it if you observe particles or color change.
- Hold the powder vial and vial adapter with one hand, firmly grasp the solvent syringe cylinder with the other, and unscrew the syringe from the vial adapter by turning it counterclockwise (Figure H).
- If you store OBIZUR at room temperature, use it immediately and within 3 hours after reconstitution.
Figure A | Figure B | Figure C | Figure D |
|
|
|
|
Figure E | Figure F | Figure G | Figure H |
|
|
|
|
Administration
For intravenous injection only!
- Before administering the reconstituted OBIZUR solution, visually inspect it for particulate matter or color change. The solution should be clear and colorless. Do not administer it if you observe particles or color change.
- Do not administer OBIZUR in the same tube or container as other injectable medicinal products.
Using an aseptic technique, administer the solution following the procedure below:
- Once all vials are reconstituted, connect a large syringe to the vial adapter by pressing the syringe cone against the Luer connector in the center of the vial adapter and turning it clockwise until the syringe is securely attached.
- Turn the vial upside down; expel the air from the syringe into the vial and withdraw the reconstituted OBIZUR solution into the syringe (Figure I).
Figure I
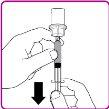
- Unscrew the large syringe from the vial adapter by turning it counterclockwise and repeat the process with all reconstituted OBIZUR vials until the total volume to be administered is reached.
- Administer the reconstituted OBIZUR solution intravenously at a rate of 1 to 2 ml per minute.
The initial dose of OBIZUR required for a patient is calculated using the following formula:
Initial dose (U/kg) = concentration of the product (U/vial) × body weight (kg) = number of vials
For example, the number of vials for the initial dose in a 70 kg subject is calculated as follows:
200 U/kg × 500 U/vial × 70 kg = 28 vials
Dosage
The recommended initial dose is 200 U per kilogram of body weight, administered by intravenous injection.
Type of Bleeding | Minimum Desired Factor VIII Activity (Units per dl or % of normal) | Initial Dose (Units per kg) | Next Dose | Frequency and Duration of Next Doses |
Mild to moderate superficial muscle bleeding without neurovascular involvement and joint bleeding | > 50% | 200 | Adjust subsequent doses based on clinical response and to maintain the minimum desired factor VIII activity | Administer doses at intervals of 4 to 12 hours; frequency may be adjusted based on clinical response and factor VIII activity |
Major muscle, retroperitoneal, gastrointestinal, intracranial bleeding of moderate to severe intensity | > 80% |
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to O-bi-zur 500 units powder and solvent for injectable solutionDosage form: INJECTABLE, 1,000 IUActive substance: coagulation factor VIIIManufacturer: Takeda Manufacturing Austria AgPrescription requiredDosage form: INJECTABLE, 1500 IUActive substance: coagulation factor VIIIManufacturer: Takeda Manufacturing Austria AgPrescription requiredDosage form: INJECTABLE, 1000 IU - after reconstitution in 2 ml of water for injections, the dose is 500 IU/mlActive substance: coagulation factor VIIIManufacturer: Takeda Manufacturing Austria AgPrescription required
Online doctors for O-bi-zur 500 units powder and solvent for injectable solution
Discuss questions about O-bi-zur 500 units powder and solvent for injectable solution, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions