NPLATE 250 micrograms POWDER AND SOLVENT FOR INJECTABLE SOLUTION

How to use NPLATE 250 micrograms POWDER AND SOLVENT FOR INJECTABLE SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Nplate 250 micrograms powder and solvent for solution for injection
Nplate 500 micrograms powder and solvent for solution for injection
romiplostim
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Nplate and what is it used for
- What you need to know before you use Nplate
- How to use Nplate
- Possible side effects
- Storing Nplate
- Contents of the pack and other information
- Instructions for preparing and administering an injection of Nplate
1. What is Nplate and what is it used for
The active substance in Nplate is romiplostim, which is a protein used to treat low platelet counts in patients with primary immune thrombocytopenia (ITP). ITP is a disease in which the immune system destroys its own platelets. Platelets are the blood cells that help the body to stop bleeding and form blood clots. Very low platelet counts can cause bruising and serious bleeding.
Nplate is used in adult patients (18 years or older) with ITP who have had their spleen removed and who have been previously treated with corticosteroids or immunoglobulins that have not worked.
Nplate works by stimulating the bone marrow (the part of the bone that produces blood cells) to produce more platelets. This should help to prevent bruising and bleeding related to ITP.
2. What you need to know before you use Nplate
Do not use Nplate
- if you are allergic to romiplostim or any of the other ingredients of this medicine (listed in section 6).
- if you are allergic to other medicines produced using DNA technology that uses the microorganism Escherichia coli(E. coli).
Warnings and precautions
- If you stop taking Nplate, it is likely that you will have a low platelet count again (thrombocytopenia). If you stop taking Nplate, your platelet count will need to be monitored, and your doctor will discuss the necessary precautions with you.
- If you have a risk of blood clots or if blood clots are common in your family. The risk of blood clots may also be increased if:
- you have liver problems;
- you are an elderly person (≥ 65 years);
- you are bedridden;
- you have cancer;
- you are taking birth control pills or hormone replacement therapy;
- you have recently had surgery or have had an injury;
- you are obese (overweight);
- you are a smoker.
Talk to your doctor, pharmacist, or nurse before you start using Nplate.
If you have a very high platelet count, you may have an increased risk of blood clots. Your doctor will adjust your dose of Nplate to make sure that your platelet count is not too high.
Changes in the bone marrow (increase in reticulin and possible fibrosis in the bone marrow)
Long-term use of Nplate may cause changes in your bone marrow. These changes may lead to abnormal blood cells or a lower number of blood cells being produced. A mild form of these changes in the bone marrow is called "increase in reticulin" and has been seen in clinical trials of Nplate. It is not known whether this could progress to a more severe form called "fibrosis". In your blood tests, signs of changes in the bone marrow such as abnormalities may appear. Your doctor will decide whether an abnormal blood test means that a bone marrow test should be done or whether Nplate treatment should be stopped.
Worsening of blood cancer
Your doctor may decide to do a bone marrow biopsy if they think it is necessary to make sure that you have ITP and not another disease such as Myelodysplastic Syndrome (MDS). If you have MDS and receive Nplate, you may have an increase in blast cell count and worsening of MDS up to progression to acute myeloid leukemia, which is a type of blood cancer.
Loss of response to romiplostim
If you lose response to romiplostim or are unable to maintain a platelet response during treatment with romiplostim, your doctor will investigate the reasons, including whether you have an increase in bone marrow fibers (reticulin) or whether you have developed antibodies that neutralize the activity of romiplostim.
Children and adolescents
Nplate is not recommended for use in children under 18 years.
Other medicines and Nplate
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicines.
If you are also taking other medicines that prevent blood clots (anticoagulant or antiplatelet treatment), there is a higher risk of bleeding. Your doctor will discuss this with you.
If you are taking corticosteroids, danazol, and/or azathioprine, which you may be taking to treat your ITP, you may need to reduce or stop taking them when combined with Nplate.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctor or pharmacist for advice before using this medicine. Nplate should not be used during pregnancy unless your doctor recommends it.
It is not known whether romiplostim is excreted in human milk. Nplate should not be used during breastfeeding. The decision to stop breastfeeding or stop romiplostim treatment should be made taking into account the benefit of breastfeeding to the baby and the benefit of romiplostim treatment to the mother.
Driving and using machines
Ask your doctor before driving or using machines, as some of the side effects (e.g., temporary episodes of dizziness) may affect your ability to perform these activities safely.
3. How to use Nplate
Nplate must be administered under the direct supervision of a doctor who can accurately control the amount of Nplate administered.
Nplate is given once a week by injection under the skin (subcutaneously).
The initial dose is 1 microgram of Nplate per kilogram of body weight once a week. Your doctor will tell you how much Nplate to use. Nplate should be injected once a week to maintain platelet counts. Your doctor will take blood samples regularly to evaluate how your platelets are responding and adjust the dose if necessary.
Once your platelet count is controlled, your doctor will continue to take blood samples to monitor your response. Your dose may be adjusted later to maintain long-term control of your platelet count.
Always follow your doctor's instructions for administering Nplate exactly. If you are unsure, ask your doctor again how to use Nplate.
Instructions for preparing and administering an injection of Nplate
After proper training, your doctor may also allow you to self-inject Nplate. Please read the instructions at the end of this leaflet on how to inject Nplate, as discussed with your doctor. If your doctor has allowed you to self-inject, you must follow up with them every month to determine if Nplate is working or if you should consider another treatment.
After the first month of self-injecting Nplate, you will need to demonstrate that you can still prepare and inject Nplate correctly.
If you use more Nplate than you should
Your doctor will make sure you receive the right amount of Nplate. If you have received more Nplate than you should, you may not have any physical symptoms, but your blood platelet levels may become very high, and this may increase the risk of blood clots. Therefore, if your doctor suspects that you have received more Nplate than you should, it is recommended that you be monitored for any signs or symptoms of side effects and that you receive appropriate treatment immediately.
If your doctor has allowed you to self-inject and you use more Nplate than you should, inform your doctor immediately.
If you use less Nplate than you should
Your doctor will make sure you receive the right amount of Nplate. If you have received less Nplate than you should, you may not have any physical symptoms, but your blood platelet levels may become low, and this may cause a risk of bleeding. Therefore, if your doctor suspects that you have received less Nplate than you should, it is recommended that you be monitored for any signs or symptoms of side effects and that you receive appropriate treatment immediately.
If your doctor has allowed you to self-inject and you use less Nplate than you should, inform your doctor immediately.
If you forget to use Nplate
If you miss a dose of Nplate, your doctor will tell you when you should receive the next dose.
If your doctor has allowed you to self-inject and you miss an injection, inform your doctor immediately.
If you stop using Nplate
If you stop using Nplate, it is likely that you will have a low platelet count again (thrombocytopenia). Your doctor will decide whether you should stop taking Nplate.
Self-injecting Nplate
Your doctor may decide that it is better for you to inject Nplate yourself. Your doctor, nurse, or pharmacist will show you how to inject Nplate. Do not attempt to inject yourself if you have not been trained. It is very important that you prepare Nplate correctly and take the correct dose (see section 7. Instructions for preparing and administering an injection of Nplate, at the end of this leaflet).
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Very common: may affect more than 1 in 10 people
- headache;
- allergic reaction;
- upper respiratory tract infection.
Common: may affect up to 1 in 10 people
- change in the bone marrow, including an increase in bone marrow fibers (reticulin);
- difficulty sleeping (insomnia);
- dizziness;
- tingling or numbness of the hands or feet (paresthesia);
- migraine;
- redness of the skin (flushing);
- blood clot in an artery of the lung (pulmonary embolism);
- nausea;
- diarrhea;
- abdominal pain;
- indigestion (dyspepsia);
- constipation;
- itching of the skin (pruritus);
- bleeding under the skin (ecchymosis);
- bruising (contusion);
- rash;
- joint pain (arthralgia);
- muscle pain or weakness (myalgia);
- pain in hands and feet;
- muscle spasms;
- back pain;
- bone pain;
- fatigue;
- reaction at the injection site;
- swelling of the hands and feet (peripheral edema);
- flu-like symptoms;
- pain;
- weakness (asthenia);
- fever (pyrexia);
- chills;
- bruising;
- swelling of the face, lips, mouth, tongue, or throat that can cause difficulty swallowing or breathing (angioedema);
- gastroenteritis;
- palpitations;
- inflammation of the sinuses (sinusitis);
- inflammation of the airways (bronchitis);
- blood clot in the veins (deep vein thrombosis).
Common: may affect up to 1 in 10 people (may be seen in blood or urine tests)
- low platelet count in the blood (thrombocytopenia) and low platelet count in the blood (thrombocytopenia) after stopping treatment with Nplate;
- high platelet count (thrombocytosis);
- anemia.
Uncommon: may affect up to 1 in 100 people
- change in the bone marrow; bone marrow disorder that causes scarring (myelofibrosis); enlargement of the spleen (splenomegaly); vaginal bleeding (vaginal hemorrhage), rectal bleeding (rectal hemorrhage); mouth bleeding (oral hemorrhage); bleeding at the injection site (hemorrhage at the injection site);
- heart attack (myocardial infarction); increased heart rate;
- dizziness or spinning sensation (vertigo);
- eye problems including: bleeding in the eyes (conjunctival hemorrhage); difficulty focusing or blurred vision (visual accommodation disorder, papilledema, or eye disorder); blindness; itching of the eyes (ocular pruritus); increased tearing (increased lacrimation); or visual disturbances;
- digestive system problems including: vomiting; bad breath (halitosis); difficulty swallowing (dysphagia); indigestion or stomach acid (gastroesophageal reflux disorder); blood in the stool (hematochezia); stomach upset; mouth ulcers or mouth blisters (stomatitis); tooth discoloration (tooth discoloration);
- weight loss; weight gain; alcohol intolerance; loss of appetite (anorexia or decreased appetite); dehydration;
- general feeling of being unwell (malaise); chest pain; irritability; swelling of the face (facial edema); feeling of heat; increased body temperature; feeling nervous;
- flu; localized infection; inflammation of the nasal passages and throat (nasopharyngitis);
- problems with the nose and throat including: cough; runny nose (rhinorrhea); dry throat; shortness of breath or difficulty breathing (dyspnea); nasal congestion; painful breathing (painful respiration);
- joint pain and swelling caused by uric acid (gout);
- muscle stiffness; muscle weakness; shoulder pain; muscle contractions;
- nervous system problems including: involuntary muscle contractions (clonus); distorted sense of taste (dysgeusia); decreased sense of taste (hypogeusia); feeling of decreased sensitivity, especially in the skin (hypoesthesia); nerve problems in the arms and legs (peripheral neuropathy); blood clot in the transverse sinus (transverse sinus thrombosis);
- depression; abnormal dreams;
- hair loss (alopecia); sensitivity to light (photosensitivity reaction); acne; allergic skin reaction to contact with allergens (contact dermatitis); skin manifestations with rash and blisters (eczema); dry skin; redness of the skin (erythema); severe scaling or blistering skin condition (exfoliative rash); abnormal hair growth; thickening and itching of the skin from repeated scratching (prurigo); bleeding under the skin surface or bruising under the skin (purpura); itchy skin rash (papular rash); itchy skin rash (pruriginous rash); generalized itchy rash (urticaria); skin nodule; abnormal skin odor (abnormal skin odor);
- circulation problems including: blood clots in the liver vein (portal vein thrombosis); low blood pressure (hypotension); high blood pressure; blockage of a blood vessel (peripheral embolism); reduced blood flow to the hands, ankles, or feet (peripheral ischemia); swelling and clotting in a vein, which can be extremely soft to the touch (superficial thrombophlebitis or phlebitis); blood clotting (thrombosis);
- a rare condition characterized by periods of burning pain, redness, and heat in the hands and feet (erythromelalgia).
Uncommon: may affect up to 1 in 100 people (may be seen in blood or urine tests)
- a rare type of anemia in which the red blood cells, white blood cells, and platelets are reduced in number (aplastic anemia);
- increase in the number of white blood cells (leukocytosis);
- excess production of platelets (thrombocythemia); increase in platelet count; abnormal platelet count;
- changes in some blood tests (increased transaminases; increased lactate dehydrogenase in the blood);
- or cancer of the white blood cells (multiple myeloma);
- proteins in the urine.
Reporting of side effects
If you experience any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storing Nplate
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the carton and on the label of the vial after EXP. The expiry date refers to the last day of the month shown.
Store in a refrigerator (between 2°C and 8°C).
Do not freeze.
Store in the original package to protect from light.
When stored in the original package, this medicine can be kept out of the refrigerator for a maximum of 30 days at room temperature (up to 25°C).
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Container Contents and Additional Information
Nplate Composition
- The active ingredient is romiplostim.
Each vial of Nplate 250 micrograms powder for solution for injection contains a total of 375 micrograms of romiplostim. An additional volume has been added to each vial to ensure that 250 micrograms of romiplostim can be administered. After dissolution, a final volume of 0.5 ml of solution contains 250 micrograms of romiplostim (500 micrograms/ml).
Each vial of Nplate 500 micrograms powder for solution for injection contains a total of 625 micrograms of romiplostim. An additional volume has been added to each vial to ensure that 500 micrograms of romiplostim can be administered. After dissolution, a final volume of 1 ml of solution contains 500 micrograms of romiplostim (500 micrograms/ml).
- The other components are:
Powder: mannitol (E421), sucrose, L-histidine, hydrochloric acid (for pH adjustment), and polysorbate 20.
Solvent: water for injections.
Product Appearance and Container Contents
Nplate is a white powder for solution for injection supplied in a 5 ml single-dose glass vial.
Nplate is supplied in a pack or multipack containing 4 packs. Each pack contains:
1 vial of 250 micrograms or 500 micrograms of romiplostim.
1 pre-filled syringe containing 0.72 or 1.2 ml of water for injections for reconstitution.
1 syringe plunger.
1 sterile vial adapter.
1 sterile 1 ml syringe with Luer lock.
1 sterile safety needle.
4 alcohol swabs.
Only certain pack sizes may be marketed.
Marketing Authorisation Holder and Manufacturer
Amgen Europe B.V.
Minervum 7061
4817 ZK Breda
Netherlands
Marketing Authorisation Holder
Amgen Europe B.V.
Minervum 7061
4817 ZK Breda
Netherlands
Manufacturer
Amgen Technology (Ireland) Unlimited Company
Pottery Road
Dun Laoghaire
Co. Dublin
Ireland
Manufacturer
Amgen NV
Telecomlaan 5-7
1831 Diegem
Belgium
You can request more information about this medicinal product from the local representative of the marketing authorisation holder.
België/Belgique/Belgien s.a. Amgen n.v. Tel: +32 (0)2 7752711 | Lietuva Amgen Switzerland AG Vilniaus filialas Tel: +370 5 219 7474 |
| Luxembourg/Luxemburg s.a. Amgen Belgique/Belgien Tel: +32 (0)2 7752711 |
Ceská republika Amgen s.r.o. Tel: +420 221 773 500 | Magyarország Amgen Kft. Tel.: +36 1 35 44 700 |
Danmark Amgen filial af Amgen AB, Sverige Tlf: +45 39617500 | Malta Amgen S.r.l. Italy Tel: +39 02 6241121 |
Deutschland Amgen GmbH Tel.: +49 89 1490960 | Nederland Amgen B.V. Tel: +31 (0)76 5732500 |
Eesti Amgen Switzerland AG Vilniaus filialas Tel: +372 586 09553 | Norge Amgen AB Tlf: +47 23308000 |
| Österreich Amgen GmbH Tel: +43 (0)1 50 217 |
España Amgen S.A. Tel: +34 93 600 18 60 | Polska Amgen Biotechnologia Sp. z o.o. Tel.: +48 22 581 3000 |
France Amgen S.A.S. Tél: +33 (0)9 69 363 363 | Portugal Amgen Biofarmacêutica, Lda. Tel: +351 21 4220606 |
Hrvatska Amgen d.o.o. Tel: +385 (0)1 562 57 20 | România Amgen România SRL Tel: +4021 527 3000 |
Ireland Amgen Ireland Limited Tel: +353 1 8527400 | Slovenija AMGEN zdravila d.o.o. Tel: +386 (0)1 585 1767 |
Ísland Vistor hf. Sími: +354 535 7000 | Slovenská republika Amgen Slovakia s.r.o. Tel: +421 2 321 114 49 |
Italia Amgen S.r.l. Tel: +39 02 6241121 | Suomi/Finland Amgen AB, sivuliike Suomessa/Amgen AB, filial i Finland Tel: +358 (0)9 54900500 |
Kúπρος C.A. Papaellinas Ltd Τηλ.: +357 22741 741 | Sverige Amgen AB Tel: +46 (0)8 6951100 |
Latvija Amgen Switzerland AG Rigas filiale Tel: +371 257 25888 |
Date of Last Revision of this Leaflet
Other Sources of Information
Detailed information on this medicinal product is available on the European Medicines Agency web site http://www.ema.europa.eu
- Instructions for Preparation and Administration of a Nplate Injection
This section contains information about how to self-inject Nplate. It is important that you do not attempt to self-administer the injection if you have not received training from your doctor, nurse, or pharmacist. If you have questions about how to give the injection, ask your doctor, nurse, or pharmacist. It is very important that the product is prepared correctly and that the administered dose is correct.
This section is divided into the following sub-sections:
Before You Start
Step 1. Place the Injection Materials
Step 2. Prepare the Vial for Use, Place the Vial Adapter
Step 3. Prepare the Sterile Water Syringe
Step 4. Dissolve Nplate by Injecting Water into the Vial
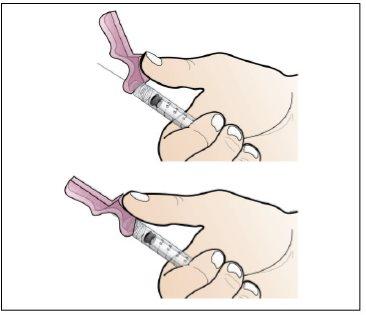
Step 5. Prepare a New Syringe for Injection
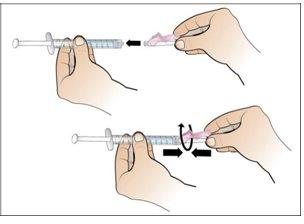
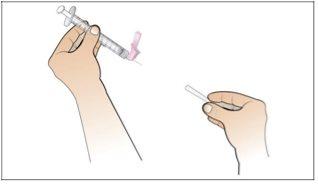
Step 6. Prepare the Needle for Injection
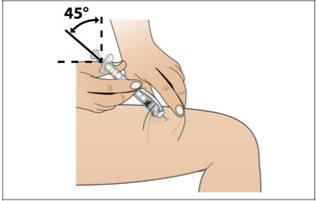
Step 7. Choose and Prepare the Injection Site
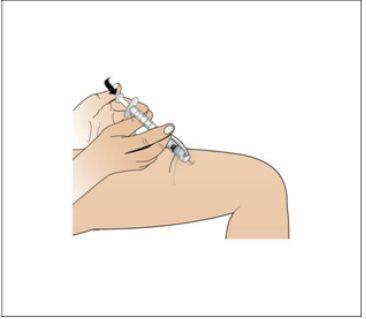
Step 8. Inject the Nplate Liquid
Step 9. Disposal of Supplies
Before You Start
Read all the instructions carefully.These instructions are for patients who have already been trained by their healthcare professional, such as their doctor, nurse, or pharmacist, in self-injection. If you have not been trained, please contact your healthcare professional.
The Nplate self-injection kit should be kept in the original packaging until use to protect the Nplate vial from light. Keep the Nplate self-injection kit refrigerated between 2°C and 8°C.
Once Nplate has been dissolved, inject immediately.
It is possible that there will be excess Nplate after administration of the prescribed dose. Do not reuse Nplate!Any excess dissolved Nplate should be discarded immediately after completing the injection process. The leftover Nplate from the vial must neverbe reused for another injection.
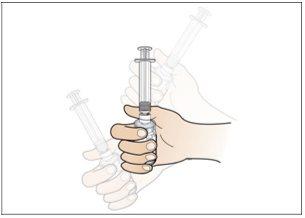
Step 1. Place the Injection Materials
Do the following:
- Select a flat and well-lit work surface, such as a table.
- Take the Nplate self-injection kit out of the refrigerator. Do not use if it is frozen.If you have any doubts about storage, contact your healthcare professional for further instructions.
Check the expiration date on the self-injection kit.If the expiration date has passed, do not use it. Do not continue and inform your healthcare professional.
- Note:If your doctor has instructed you that the Nplate dose requires more than one injection, you will need to use more than one self-injection kit. Follow the steps described in this leaflet and use as many self-injection kits as necessary to complete the prescribed dose of Nplate.
- Make sure you have the following components:
Alcohol swab pack x4

1 vial of 250 micrograms or | Vial adapter 13 mm x1 |
500 micrograms x1 | |

Plunger for pre-filled syringe of sterile water x1Pre-filled syringe of sterile water x1

1 ml syringe with Luer lock x1Safety needle for injection x1

- Do notopen the components until instructed to do so in the instructions.
- Do notuse altered or damaged components.
- Do notreuse components.
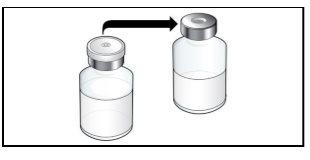
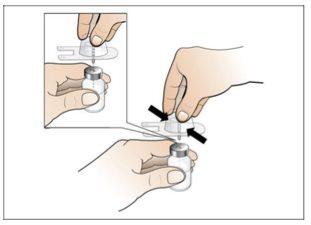
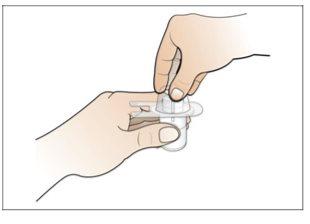
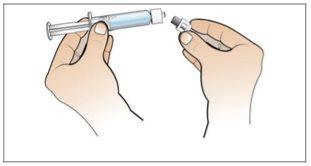
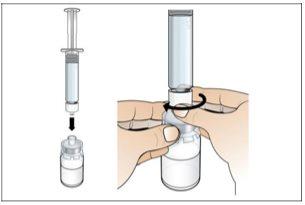
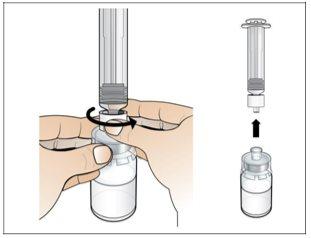
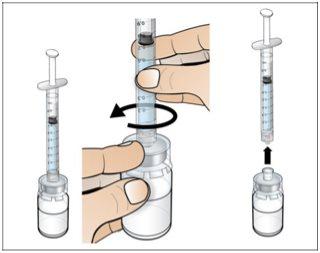
Step 2. Prepare the Vial for Use, Place the Vial Adapter
Use:2 alcohol swab packs, 1 vial, and 1 vial adapter pack.
Do the following:
(500 micrograms) from the vial. |
|
|
|
|
|
|
|
|
|
|
|
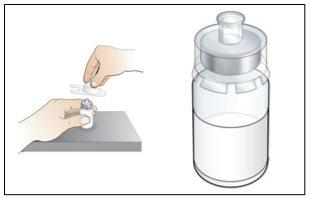
Step 3. Prepare the Sterile Water Syringe
Use:The pre-filled syringe of sterile water and the plunger.
Before starting Step 3, please note the following:
- The transparent plastic plunger must alwaysbe connected before breaking the white tip of the pre-filled syringe of sterile water. Perform step 3a before step 3b.
Do the following:
|
|
|
|
|
|
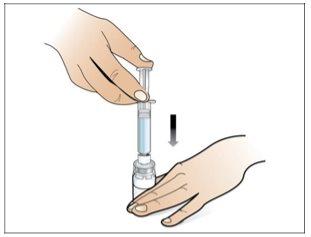
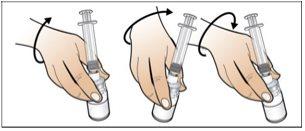
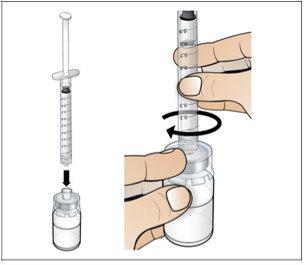
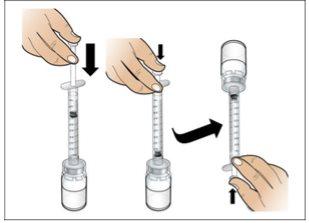
Step 4. Dissolve Nplate by Injecting Water into the Vial
Use:The pre-filled syringe of sterile water and the vial with the vial adapter attached.
Before starting Step 4, please note the following:
- Dissolveslowly and carefully. It is a protein and, as such, can be easily damaged by inadequate and excessive mixing.
Do the following:
|
|
|
Press slowly and gently |
Before continuing:
- Make surethat all the water is injected from the syringe into the vial before dissolving.
|
Correct |
|
Incorrect |
Before continuing:
- Visually inspectthe dissolved liquid for particles and/or discoloration. It should be clear and colorless and completely dissolved.
- Note:If there is any color or particles in the liquid,contact your healthcare professional.
- Make surethat the liquid is completely dissolved before removing the syringe.
|
|
- Discard the empty syringein a puncture-proof container. Keep the dissolved Nplate vial. Immediately prepare a new syringe for injection.
- Do notdelay the injection of Nplate
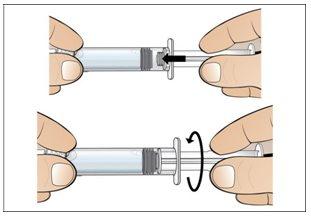
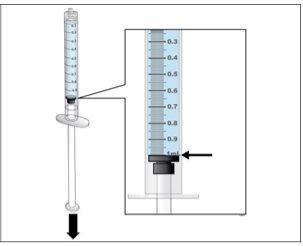
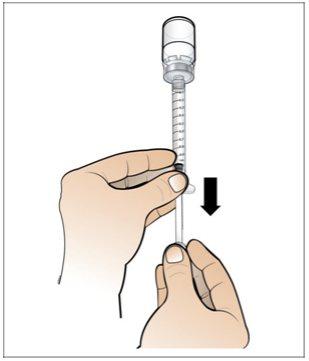
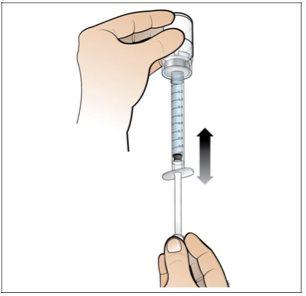
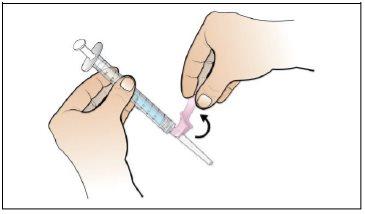
Step 5. Prepare a New Syringe for Injection
Use:A new 1 ml syringe from the packaging and the vial with the dissolved and transparent Nplate.
Before continuing:
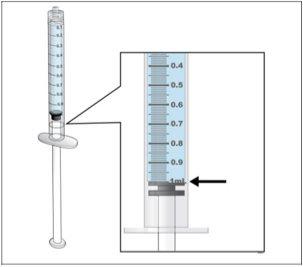
- Reviewyour dose before starting this step.
- Note:Nplate liquid is very potent, so dose accuracy and measurement are important.
- Make surethat all air bubbles have been removed before injection.
Do the following:
|
|
Draw air into the syringe up to the 1 ml mark | |
|
|
|
Turn downwards |
|
|
|
Correct |
|
Air bubbles: Correct Incorrect |
|
Adjust the amount to your prescribed dose |
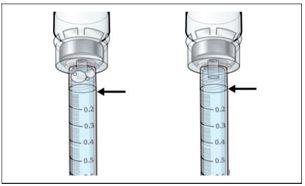
- Perform a final check to ensure that the correct amount of liquid for your dose is in the syringeand that all air bubbles have been removed.
Before proceeding:
- Ensurethat the correct amount of liquid for your dose remains in the syringe.
- Ensurethat all air bubbles are removed from the syringe.
|
|
|
|
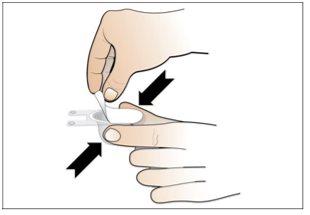
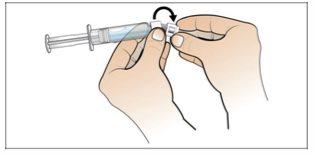
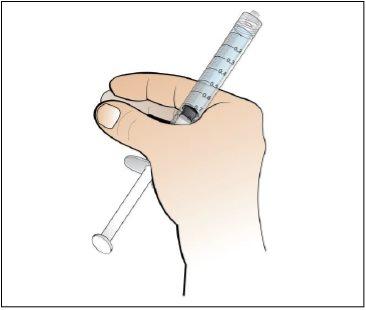
Step 6. Prepare the needle for injection
Using:The syringe filled with the measured dose of Nplate and the safety needle.
Do the following:
|
|
|
|
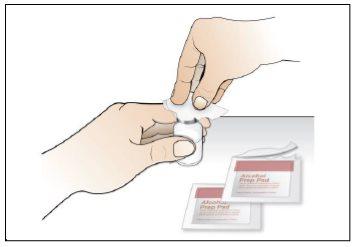
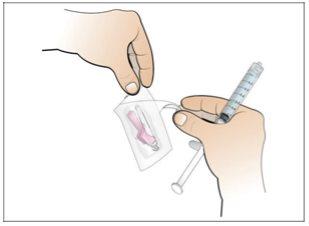
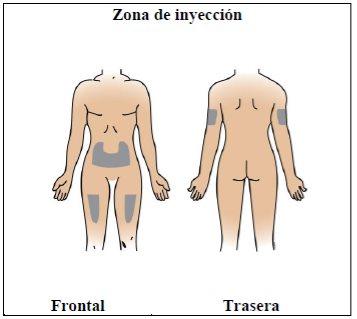
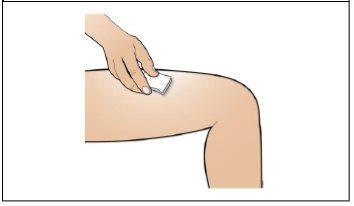
Step 7. Choose and prepare the injection site
Using:New alcohol swabs.
Do the following:
|
|
|
|
Step 8. Inject the Nplate liquid
Using:The syringe filled with the measured dose and the needle.
Do the following:
|
|
|
|
|
|
|
|
|
|
|
|
Step 9. Disposal of supplies
Do the following:
- Immediately discard the syringe with the covered needleinto a puncture-resistant container.
- Immediately discard the used Nplate vialinto an appropriate waste container.
- Ensure that all remaining materials are discarded in the appropriate containers.
The Nplate injection device and vial NEVERshould be reused.
- Discardthe used needle and syringe in a puncture-resistant container.
Discardany remaining Nplate in an appropriate waste container. Any leftover Nplate from a vial NEVER can be reused for another injection.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to NPLATE 250 micrograms POWDER AND SOLVENT FOR INJECTABLE SOLUTIONDosage form: INJECTABLE, 250 µgActive substance: romiplostimManufacturer: Amgen Europe B.V.Prescription requiredDosage form: INJECTABLE, 500 µgActive substance: romiplostimManufacturer: Amgen Europe B.V.Prescription requiredDosage form: INJECTABLE, 500 µgActive substance: romiplostimManufacturer: Amgen Europe B.V.Prescription required
Online doctors for NPLATE 250 micrograms POWDER AND SOLVENT FOR INJECTABLE SOLUTION
Discuss questions about NPLATE 250 micrograms POWDER AND SOLVENT FOR INJECTABLE SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions