
NICORETTE BUCOMIST 1 mg/puff ORAL SPRAY FRUIT MINT FLAVOR


How to use NICORETTE BUCOMIST 1 mg/puff ORAL SPRAY FRUIT MINT FLAVOR
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Nicorette BucoMist 1mg/puff Oral Spray Fruit Mint Flavor
Nicotine
Read the entire package leaflet carefully before starting to use this medication, as it contains important information for you.
Follow the administration instructions for the medication contained in this package leaflet or as indicated by your doctor or pharmacist.
- Keep this package leaflet, as you may need to read it again.
- If you need advice or more information, consult your pharmacist.
- If you experience side effects, consult your doctor or pharmacist, even if they are side effects not listed in this package leaflet. See section 4.
- You should consult a doctor if your condition worsens or does not improve after 6 months of treatment with Nicorette BucoMist.
Contents of the Package Leaflet
- What is Nicorette BucoMist and what is it used for
- What you need to know before starting to use Nicorette BucoMist
- How to use Nicorette BucoMist
- Possible side effects
- Storage of Nicorette BucoMist
- Package contents and additional information
1. What is Nicorette BucoMist and what is it used for
Nicorette BucoMist belongs to the group of medications used as an aid to quit smoking when there is an intention to quit or reduce tobacco consumption before definitively abandoning it. This type of treatment is called Nicotine Replacement Therapy (NRT).
Nicorette BucoMist alleviates the withdrawal symptoms that appear when quitting smoking, including the urge to smoke. When nicotine from tobacco is suddenly stopped from being administered to the body, various unpleasant sensations may appear, which are collectively called withdrawal syndrome. The use of Nicorette BucoMist can prevent or reduce withdrawal syndrome, including the urge to smoke. Its action is due to the fact that Nicorette BucoMist continues to administer a small amount of nicotine to the body for a short period. Nicorette BucoMist does not contain tar, carbon monoxide, or other toxins present in tobacco.
To increase the chances of quitting smoking, it is essential to have advice and support.
You should consult a doctor if your condition worsens or does not improve after 6 months of treatment.
2. What you need to know before starting to use Nicorette BucoMist
Do not use Nicorette BucoMist
- If you are allergic to nicotine or any of the other components of this medication (listed in section 6).
- If you are under 18 years old.
- If you have never smoked.
Warnings and precautions
Consult your doctor or pharmacist before starting to use Nicorette BucoMist.
Consult your doctor before starting to use this medication if you suffer from any of the following diseases. It is possible that you can use Nicorette BucoMist, but you will need to consult your doctor first:
- If you have recently (up to 3 months prior) suffered a heart attackor stroke.
- If you suffer from chest pain(angina pectoris) or resting angina.
- If you suffer from any heart diseasethat affects heart rhythm or frequency.
- If you have high blood pressure(hypertension) that is not controlled with medication.
- If you have ever had an allergic reactionwith inflammation of the lips, face, and throat (angioedema) or skin irritation (urticaria). The use of nicotine replacement therapy may sometimes produce this type of reaction.
- If you suffer from severe or moderate liver disease.
- If you suffer from severe kidney disease.
- If you suffer from diabetes.
- If you have hyperactivity of the thyroid gland.
- If you have a tumor in the adrenal medulla(pheochromocytoma).
- If you suffer from stomach or duodenal ulcers.
- If you have esophagitis.
- If you have a history of epilepsy or seizures
Non-smokersshould not use Nicorette BucoMist.
Children and adolescents
Do not administer this medication to children and adolescents.
Using Nicorette BucoMist with other medications
Inform your doctor or pharmacist that you are taking, have recently taken, or may need to take any other medication. This is especially important if you take medications that contain:
Theophyllinefor asthma
Tacrinefor Alzheimer's disease
Clozapinefor schizophrenia
Ropinirolefor Parkinson's disease
Using Nicorette BucoMist with food and drinks
Do not eat or drink when applying a spray.
Pregnancy, breastfeeding, and fertility
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medication.
Pregnancy
It is very important to quit smoking during pregnancybecause it could cause intrauterine growth retardation, premature birth, or stillbirth. Ideally, you should try to quit smoking without using any nicotine medication. If you cannot, you should only use Nicorette BucoMist if your primary care physician, obstetrician, or a specialist in smoking cessation recommends it.
Breastfeeding
The use of Nicorette BucoMist should be avoided during breastfeeding, as nicotine passes into breast milk in amounts that may affect the baby. If your doctor has recommended using Nicorette BucoMist during breastfeeding, the spray should be applied immediately after breastfeeding and never during the 2 hours prior to breastfeeding.
Fertility
Smoking increases the risk of infertility in women and men. The effect of nicotine on fertility is unknown.
Driving and using machines
No effects on the ability to drive or use machines have been observed.
Nicorette BucoMistcontains 12 mg of propylene glycol per spray. This medication contains approximately 7 mg of alcohol (ethanol) in each spray, which is equivalent to 97 mg/ml. The amount of alcohol in each spray of this medication is equivalent to less than 2 ml of beer or 1 ml of wine. The small amount of alcohol in this medication will not have any noticeable effect. This medicinal product also contains less than 1 mmol of sodium (23 mg) per spray, i.e., it is essentially sodium-free. Due to the presence of butylhydroxytoluene, Nicorette BucoMist may cause skin reactions (e.g., contact dermatitis) or irritation to the eyes or mucous membranes.
.
3. How to use Nicorette BucoMist
Follow the administration instructions for the medication contained in this package leaflet or as indicated by your doctor or pharmacist. In case of doubt, ask your doctor or pharmacist.
Nicorette BucoMist should not be used in individuals under 18 years old.
Consult your doctor if you have not achieved a reduction in the number of sprays or cigarette consumption after 6 weeks of treatment or if you need to use Nicorette BucoMist for more than 6 months. Normally, you should use Nicorette BucoMist for 3 months. Nicorette BucoMist should not be taken for more than 6 months.
The method of using Nicorette BucoMist will depend on whether you quit smoking immediately or gradually reduce the number of cigarettes before quitting completely.
Quitting smoking immediately
The goal is to quit smoking immediately and use the spray to alleviate the urge to smoke.
Do not use more than 2 sprays at a time or 4 sprays per hour for 16 hours. The maximum dose is 64 sprays during 16 hours in any 24-hour period.
Phase 1:Weeks 1-6 Perform 1 or 2 sprays when the urge to smoke appears. If after one spray the urge to smoke is not controlled within a few minutes, apply a second spray. If two sprays are needed, subsequent applications should also be two consecutive sprays. For most smokers, this means 1 or 2 sprays every 30-60 minutes. Example: If you smoke an average of 15 cigarettes per day, you should apply 1-2 sprays, at least 15 times a day. Phase 2:Weeks 7-9 Start reducing the number of sprays per day. By the end of the ninth week, you should be using HALF the number of sprays you used during Phase 1. Phase 3:Weeks 10-12 Continue reducing the number of sprays per day until you use no more than 4 sprays per day during the twelfth week. When you have reduced the sprays to 2-4 sprays per day, you should stop using Nicorette BucoMist. |
Quitting smoking gradually
The goal is to start replacing some cigarettes with Nicorette BucoMist. Once this goal is achieved, quit smoking cigarettes completely and switch to using the spray only.
When you feel a strong urge to smoke, apply 1-2 sprays of the spray instead of a cigarette to help control your cravings. The spray is to replace a cigarette, do not smoke shortly after using it. Using the spray without reducing the number of cigarettes will make you feel sick (see section "If you use more Nicorette BucoMist than you should"). Reduce the number of cigarettes you smoke per day as much as possible and replace them with a spray. As soon as you feel ready, you should quit smoking completely, in any case no later than 12 weeks after starting treatment. After quitting smoking, gradually reduce the number of sprays per day. When you have reduced the sprays to 2-4 per day, stop using Nicorette BucoMist.
Do not use more than 2 sprays at a time or 4 sprays per hour for 16 hours. The maximum dose is 64 sprays during 16 hours in any 24-hour period.
After completing the treatment, you may have the temptation to smoke again. Keep the package with the unused doses of the medication for situations where you suddenly have a strong urge to smoke. You can apply one spray or two sprays if the first spray does not help within a few minutes.
Follow these instructions carefully and use the diagrams as a guide
Open the mouthpiece


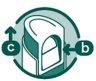
How to open the dispenser
- Use your thumb to slide the button (a) down until it can be pressed lightly inward (b). Do not press too hard.
- While pressing the button, slide up (c) to open the top of the dispenser.

How to load the dispenser
When using Nicorette BucoMist for the first time, you need to load the spray pump. Point the mouthpiece towards a safe place for you where no other adults, children, or pets are present. Press the top of the dispenser with your index finger 3 times until a fine spray appears. If you do not use Nicorette BucoMist for 2 days, the loading procedure must be repeated.
How to use the dispenser

- Point the mouthpiece towards the open mouth as close as possible.
- Press the top of the dispenser firmly to release a spray inside the mouth, avoiding the lips. Do not inhale while applying to avoid the spray entering the throat. For better results, do not swallow in the seconds following the spray.
To remove the mouthpiece

How to close the dispenser
- Slide the button (d) down until it can be pressed inward.
- While pressing, slide the top of the dispenser down (f). Release the button. The dispenser is now closed.
To use another dose, repeat the steps mentioned above.
Close the dispenser after each use to prevent the use of Nicorette BucoMist by children or accidental sprays. Be careful not to spray your eyes during the administration of Nicorette BucoMist. If the spray comes into contact with your eyes, rinse them abundantly with water.
If you use more Nicorette BucoMist than you should
If you smoke at the same time as using Nicorette BucoMist, you may experience symptoms of nicotine overdose.
If a child uses Nicorette BucoMist or if you use too many sprays, consult your doctor immediately, go to the nearest hospital, or call the Toxicology Information Service (telephone 91 562 04 20), indicating the name and amount used. The doses tolerated by adults during treatment can cause severe symptoms of poisoning in childrenand even lead to death.
The symptoms of overdose are nausea, vomiting, excessive salivation, stomach pain, diarrhea, sweating, headache, and dizziness, hearing disturbances, and a feeling of weakness. At high doses, these symptoms can be followed by a drop in blood pressure, weak and irregular pulse, difficulty breathing, extreme fatigue, circulatory collapse, and generalized convulsions.
If you have any other questions about the use of this medication, ask your doctor or pharmacist.
4. Possible side effects
Like all medications, Nicorette BucoMist can cause side effects, although not everyone will experience them.
Nicorette BucoMist may cause the same side effects as other forms of nicotine. These effects usually depend on the dose taken.
Effects related to quitting smoking (nicotine withdrawal)
Some of the unwanted effects experienced when quitting smoking may be withdrawal syndromes due to the decrease in nicotine consumption.
These effects include:
- Irritability, aggression, impatience, or frustration.
- Feeling of anxiety, agitation, or difficulty concentrating.
- Sleep disorders.
- Increased appetite or weight gain.
- Depression.
- Urgency to smoke (cravings).
- Decreased heart rate.
- Gum bleeding or mouth ulcers.
- Dizziness or mild disorientation.
- Cough, sore throat, nasal congestion, or nasal discharge.
- Constipation.
If you notice any of the following rare side effects, stop using Nicorette BucoMist and consult your doctor immediately (signs of angioedema):
- Swelling of the face, tongue, or throat.
- Difficulty swallowing.
- Urticaria or difficulty breathing.
Very common: may affect more than 1 in 10 people:
- Hiccup (particularly common).
- Headache, nausea (feeling sick).
- Throat irritation.
Common: may affect up to 1 in 10 people:
- Effects at the administration site such as burning sensation, mouth inflammation, or changes in taste perception.
- Dry mouth or increased saliva production.
- Feeling of dyspepsia.
- Pain or discomfort in the abdomen.
- Vomiting, flatulence, or diarrhea.
- Feeling of fatigue.
- Hypersensitivity (allergy)
- Numbness.
Uncommon: may affect up to 1 in 100 people:
- Effects on the nose such as nasal congestion or sneezing.
- Nasal discharge
- Wheezing (bronchospasm), or feeling the need to breathe more forcefully than normal (dyspnea), throat tightness.
- Redness of the skin (flushing) or increased sweating.
- Effects in the mouth such as numbness, tongue inflammation, mouth ulcers, damage to the mouth mucosa, or changes in voice, mouth and throat pain, belching, gum bleeding.
- Palpitations (unusual sensation of heartbeat), increased heart rate, hypertension.
- Rashes and/or itching (pruritus, urticaria) of the skin.
- Abnormal dreams.
- Chest discomfort and pain.
- Feeling of weakness, feeling of malaise.
Rare: may affect up to 1 in 1,000 people:
- Difficulty swallowing, feeling of numbness in the mouth.
- Gagging.
Frequency not known: frequency cannot be estimated from the available data
- Blurred vision, increased tear production (lacrimation).
- Dry throat, stomach discomfort, lip pain.
- Abnormal heart rhythm.
- Redness of the skin.
- Allergic reactions including swelling of the face and mouth (angioedema or anaphylaxis).
- Seizures
Reporting side effects
If you experience side effects, consult your doctor or pharmacist, even if they are side effects not listed in this package leaflet.
You can also report them directly through the Spanish Medicines Monitoring System for Human Use. Website: www.notificaram.es. By reporting side effects, you can contribute to providing more information on the safety of this medication.
5. Storage of Nicorette BucoMist
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiration date that appears on the dispenser and on the packaging after CAD. The expiration date is the last day of the month indicated.
Do not store at a temperature above 30°C.
Medicines should not be disposed of via wastewater or household waste. Deposit the packaging and any unused medicines at the SIGRE collection point in your pharmacy. If in doubt, ask your pharmacist how to dispose of the packaging and any unused medicines. This will help protect the environment, especially the aquatic environment in the case of nicotine.
6. Package Contents and Additional Information
Nicorette BucoMist Composition
- The active ingredient is nicotine. One spray releases 1 mg of nicotine.
- The other components (excipients) are propylene glycol (E1520), anhydrous ethanol, tromethamine, poloxamer 407, glycerol (E422), sodium hydrogen carbonate, levomenthol, red fruit flavoring, refreshing aroma, sucralose, potassium acesulfame, butylated hydroxytoluene (E321), hydrochloric acid, and purified water.
Product Appearance and Package Contents
Nicorette BucoMist consists of a plastic bottle located in a dispenser with a mechanical spray pump. The dispenser has a child safety system.
Each bottle contains 13.2 ml of solution, equivalent to 150 sprays.
Nicorette BucoMist is supplied in packages of 1, 2, or 3 dispensers.
 Nicorette BucoMist is also available in packages of 1, 2, or 3 dispensers that include, under the rear label, a near-field communication (NFC) chip that enables connectivity with the mobile application. These dispensers carry this icon
Nicorette BucoMist is also available in packages of 1, 2, or 3 dispensers that include, under the rear label, a near-field communication (NFC) chip that enables connectivity with the mobile application. These dispensers carry this icon
Only certain package sizes may be marketed.
Marketing Authorization Holder and Manufacturer
Marketing authorization holder:
JNTL Consumer Health (Spain), S.L.
Paseo de las Doce Estrellas 5-7
28042 Madrid, Spain
Manufacturer:
McNeil AB,
P.O. Box 941, Norrbroplatsen 2, SE-251 09 Helsingborg, Sweden
or
Johnson & Johnson Consumer NV/SA,
Michel De Braeystraat 52, 2000 Antwerp
This medicine is authorized in the member states of the European Economic Area under the following names:
Sweden | Nicorette Fruktmint |
Austria | Nicorette Fruit & Mint Spray |
Belgium | Nicorette Fruit & Mint Mondspray |
Czech Republic | Nicorette Spray s príchutí lesního ovoce |
Denmark | Nicorette QuickMist Cool Berry |
Finland | Nicorette Berrymint |
France | NICORETTESPRAY FRUITS ROUGES |
Germany | Nicorette Fruit & Mint Spray |
Ireland | Nicorette QuickMist Cool Berry |
Luxembourg | Nicorette Fruit & Mint Spray Buccal |
Norway | Nicorette |
Poland | Nicorette Cool Berry |
Portugal | Nicorette Bucomist fruit mint flavor |
Slovakia | Nicorette Spray s príchutou lesného ovocia |
Spain | Nicorette Bucomist fruit mint flavor |
Netherlands | Nicorette Fruit & Mint Mondspray |
Date of the last revision of this prospectus: June 2023
Detailed and updated information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es
- Country of registration
- Active substance
- Prescription requiredNo
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to NICORETTE BUCOMIST 1 mg/puff ORAL SPRAY FRUIT MINT FLAVORDosage form: CHEWING GUM, 2 mgActive substance: nicotineManufacturer: Enorama Pharma AbPrescription not requiredDosage form: CHEWING GUM, 4 mgActive substance: nicotineManufacturer: Enorama Pharma AbPrescription not requiredDosage form: CHEWING GUM, 2 mgActive substance: nicotineManufacturer: Enorama Pharma AbPrescription not required
Online doctors for NICORETTE BUCOMIST 1 mg/puff ORAL SPRAY FRUIT MINT FLAVOR
Discuss questions about NICORETTE BUCOMIST 1 mg/puff ORAL SPRAY FRUIT MINT FLAVOR, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions













