
MYALEPTA 5.8 mg POWDER FOR INJECTABLE SOLUTION

How to use MYALEPTA 5.8 mg POWDER FOR INJECTABLE SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Myalepta 5.8mg powder for solution for injection
metreleptin
This medicine is subject to additional monitoring, which will allow for quick identification of new safety information. You can help by reporting any side effects you may get. The last section of the leaflet includes information on how to report side effects.
Read all of this leaflet carefully before you start using this medicine, because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Myalepta and what is it used for
- What you need to know before you use Myalepta
- How to use Myalepta
- Possible side effects
- Storage of Myalepta
- Contents of the pack and further information
1. What is Myalepta and what is it used for
Myalepta contains the active substance metreleptin. Metreleptin is similar to a human hormone called leptin.
What Myalepta is used for
Myalepta is used to treat complications caused by leptin deficiency in patients with lipodystrophy.
It is used in adults, adolescents, and children 2 years of age and older:
- with generalized lipodystrophy (the entire body lacks enough fatty tissue)
It is used in cases where other treatments have not been effective in adults and adolescents 12 years of age and older:
- who have inherited partial lipodystrophy (also called familial or congenital lipodystrophy), or
- whose partial lipodystrophy is the result of their body's response to something, such as a viral disease (also called acquired lipodystrophy)
How Myalepta works
Fatty tissue produces natural leptin, which has many bodily functions, such as:
- controlling hunger and energy levels
- helping the body's insulin control blood sugar levels
Metreleptin mimics the effects of leptin. This improves the body's ability to control energy levels.
2. What you need to know before you use Myalepta
Do not use Myalepta
- if you are allergic to metreleptin or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Talk to your doctor, pharmacist, or nurse before starting Myalepta if
- you are pregnant
- you have ever had a type of cancer called lymphoma
- you have ever had blood problems (such as anemia)
- you have ever had inflammation of the pancreas ("pancreatitis")
- you have or have had problems with the immune system (autoimmune diseases, including autoimmune liver problems)
Lymphoma
People with lipodystrophy may be at risk of a type of blood cancer called lymphoma, whether or not they use Myalepta.
However, it is possible that the risk of getting lymphoma may be higher when using this medicine.
Your doctor will decide whether you should use Myalepta and will monitor you during treatment.
Severe and serious infections
During treatment with Myalepta, your body may produce antibodies that can increase the risk of developing severe or serious infections. Tell your doctor immediately if you develop a high fever, accompanied by increased tiredness (see section 4).
Hypoglycemia with insulin or other antidiabetic medicines
If you are using a medicine like insulin or other medicines to treat diabetes, your doctor will closely monitor your blood sugar levels. Your doctor may adjust the dose of insulin or other medicines if necessary.
The goal is to prevent your blood sugar levels from becoming too low ("hypoglycemia"). For signs of hypoglycemia, see section 4 "Signs of hyperglycemia and hypoglycemia".
Hyperglycemia and high blood fat levels
You may have higher blood sugar ("hyperglycemia") or fat ("hypertriglyceridemia") levels when taking Myalepta, which can be a sign that this medicine is not working as well as it should. Signs of hyperglycemia and high blood fat levels are included in section 4 "Signs of hyperglycemia and hypoglycemia" and "Signs of high blood fat levels".
If you experience any of the symptoms mentioned above, or if you are unsure, talk to your doctor immediately. Your doctor may need to change your treatment.
Autoimmune diseases
People who have or have had problems with the immune system (autoimmune diseases, including autoimmune liver problems) may experience worsening of symptoms with Myalepta. Talk to your doctor about the symptoms you may experience and which would require further testing.
Allergic reactions
You may experience an allergic reaction during treatment with Myalepta. If you experience symptoms of an allergic reaction, tell your doctor immediately. Signs of an allergic reaction can be found in section 4 under "Allergic reactions".
Fertility
Myalepta may increase fertility in women with lipodystrophy (see section "Pregnancy, breastfeeding, and fertility").
Myalepta contains sodium
This medicine contains less than 1 mmol of sodium (23 mg) per dose; this is essentially "sodium-free".
Children and adolescents
Do not give this medicine to children under 2 years of age with generalized lipodystrophy or to children under 12 years of age with partial lipodystrophy. This is because the effect of this medicine in children under these ages is unknown.
Other medicines and Myalepta
Tell your doctor if you are using, have recently used, or might use any other medicines. Myalepta may affect how other medicines work. Other medicines may also affect how Myalepta works.
Tell your doctor especially if you are taking any of the following medicines:
- hormonal contraceptives, as Myalepta may reduce their effectiveness in preventing pregnancy
- theophylline used for lung problems, such as asthma
- anticoagulant medicines (such as warfarin and phenprocoumon)
- medicines that suppress the immune system (such as cyclosporine)
- antidiabetic medicines (such as insulin or insulin secretagogues), see section 2 "Hypoglycemia with insulin or other antidiabetic medicines".
If any of the above applies to you (or if you are unsure), talk to your doctor before using Myalepta. Some medicines may need to be monitored while using Myalepta, as it may be necessary to adjust the dose of these medicines.
Pregnancy, breastfeeding, and fertility
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, talk to your doctor before using this medicine.
Do not use Myalepta if you are pregnant or may become pregnant. This is because the effect of this medicine on the fetus is unknown. Women of childbearing age must use effective non-hormonal contraceptive methods, such as condoms, during Myalepta use. Talk to your doctor about suitable contraceptive methods, as Myalepta may reduce the effectiveness of hormonal contraceptives in preventing pregnancy.
It is unknown whether Myalepta passes into breast milk. If you are breastfeeding or plan to breastfeed, talk to your doctor. You and your doctor will decide whether to continue breastfeeding while using this medicine, considering the benefit of breastfeeding for the baby and the benefit of Myalepta for the mother.
Myalepta may increase fertility in women with lipodystrophy.
Driving and using machines
Myalepta has a minor influence on the ability to drive and use machines. You may feel dizzy or tired when using this medicine. If this happens, do not drive or use tools or machines. If in doubt, talk to your doctor.
3. How to use Myalepta
Follow the instructions for administration of this medicine exactly as told by your doctor. If you are unsure, talk to your doctor again.
Myalepta is an injection given once a day under the skin ("subcutaneous injection"). This medicine is used in children from 2 years of age, adolescents, and adults with generalized lipodystrophy; it is also used in children from 12 years of age, adolescents, and adults with partial lipodystrophy.
Your doctor will monitor you or your child during use of this medicine and decide on the dose you or your child should use.
Your doctor may decide that you should self-inject the medicine. Your doctor, nurse, or pharmacist will teach you how to prepare and inject the medicine.
- Do notattempt to prepare the medicine or self-inject it if you have not been instructed on how to do so.
Amount to be injected
Over time, the dose of Myalepta may change based on how this medicine works in your specific case. The Myalepta powder is dissolved by mixing it with water for injections to produce an injection solution. Read the "Instructions for use" to find out how to prepare the solution before injection.
Your doctor will have prescribed the correct dose for you based on:
- If you weigh 40 kg or less:
- The initial dose is 0.06 mg (0.012 ml of solution) per kilogram of body weight.
- If you are a maleand weigh more than 40 kg:
- The initial dose is 2.5 mg (0.5 ml of solution).
- If you are a femaleand weigh more than 40 kg:
- The initial dose is 5 mg (1 ml of solution).
Your doctor or pharmacist will tell you the amount of solution to inject. If you are unsure of the amount of solution to inject, talk to your doctor or pharmacist before injection.
- The syringe you use to inject the medicine depends on the dose prescribed for you.
- Your pharmacist will provide you with the correct syringe to use for the injection.
- See the "Instructions for use" to find out which syringe to use.
- To calculate the amount of medicine to inject (in ml), divide the dose (in mg) by 5.
- For example, if you have been prescribed a dose of 5 mg of Myalepta, dividing 5 mg by 5 will result in 1 ml, which is the amount of solution to inject with a 1 ml syringe.
- If the dose is 1.50 mg (0.30 ml of solution) or less, you must use a 0.3 ml syringe.
- The 0.3 ml syringe will show the injection amount in "units" instead of "ml". See the "Instructions for use" (section 7) for more information on reading and using other syringes.
- To calculate the amount of solution to inject (in units), divide the dose (in mg) by 5 and multiply by 100.
If you need to inject 1 ml or more of the Myalepta solution, your doctor may instruct you to split the dose into two injections. This can help make the injections more comfortable.
You must use a clean needle and syringe for both injections.
If you are unsure of the amount of solution to inject, talk to your doctor or pharmacist before injection.
When prescribing small doses/volumes (e.g., in the case of children), the vials will be kept almost full with the product after withdrawal of the required dose. The remaining solution must be discarded after use.
If you use more Myalepta than you should
If you use more Myalepta than you should, talk to your doctor or go to the hospital immediately. Your doctor will monitor you for any side effects.
If you forget to use Myalepta
- If you forget to inject a dose, inject it as soon as possible.
- Then administer the normal dose the next day.
- Do not use a double dose to make up for forgotten doses.
If you inject less Myalepta than you should, talk to your doctor immediately. Your doctor will monitor you for any side effects.
If you stop using Myalepta
Do not stop your treatment with Myalepta without talking to your doctor. Your doctor will decide whether you should stop treatment with this medicine.
If you need to stop using Myalepta, your doctor will gradually reduce the dose over a period of two weeks before stopping it completely. Your doctor will also ask you to follow a low-fat diet.
- It is important to gradually reduce the dose over a period of two weeks, as this can help prevent a sudden increase in blood fat levels ("triglycerides").
- A sudden increase in blood triglyceride levels can cause pancreatitis. Gradually reducing the dose and following a low-fat diet may help prevent this.
Do not stop treatment with Myalepta unless your doctor tells you to.
If you have any further questions on the use of this medicine, ask your doctor.
4. Possible Adverse Effects
Like all medicines, this medicine can cause adverse effects, although not all people suffer from them.
Possible adverse effects with this medicine:
Severe Adverse Effects
If you notice any of the following severe adverse effects, inform your doctor immediately (you may need urgent medical treatment). If you cannot contact your doctor, seek urgent medical assistance:
- hypoglycemia (glucose); see section “Signs of Hyperglycemia and Hypoglycemia” below
- hyperglycemia (glucose)
- blood clot in the veins (deep vein thrombosis) - pain, swelling, heat, and redness, usually in the lower leg or thigh
- fluid in the lungs (difficulty breathing or coughing)
- drowsiness or confusion
Allergic Reactions
Consult a doctor if you notice any severe allergic reaction, including:
- breathing problems
- swelling or redness of the skin, hives
- swelling of the face, lips, tongue, or throat
- stomach pain, nausea, and vomiting
- fainting or dizziness
- severe stomach pain (abdomen)
- very rapid pulse
Inflamed Pancreas(“pancreatitis”):
Consult a doctor if you notice signs of pancreas inflammation, including:
- sudden severe stomach pain (abdomen)
- general discomfort (nausea or vomiting)
- diarrhea
Other Adverse Effects
Tell your doctor if you notice any of the following adverse effects.
Very Common (may affect more than one in ten people):
- weight loss
Common(may affect up to one in ten people):
- decreased appetite
- headache
- hair loss
- unusually long or heavy menstrual bleeding
- fatigue
- bruising, redness, itching, or hives at the injection site
- production of antibodies against metreleptin, which may increase the risk of developing severe or intense infections. You may notice fever along with increased fatigue
Frequency Not Known(frequency cannot be estimated from available data):
- flu
- lower respiratory tract infection
- diabetes
- increased appetite or excessive food intake
- faster than normal heart rate
- cough
- shortness of breath
- muscle pain (“myalgia”)
- joint pain
- swelling of hands and feet
- increase in fatty tissue
- subcutaneous swelling and bleeding at the injection site
- pain at the injection site
- itching at the injection site
- general feeling of discomfort, agitation, or pain (“general discomfort”)
- increase in blood fat (“triglycerides”) (see section “Signs of High Fat Levels” below)
- increase in “HbA1c” in blood, confirmed by tests
- weight gain
- subcutaneous swelling and bleeding (“hemorrhage”)
- hyperglycemia (see section “Signs of Hyperglycemia and Hypoglycemia” below)
Tell your doctor if you notice any of the above adverse effects.
Signs of Hyperglycemia and Hypoglycemia
Among the symptoms of hypoglycemiaare:
- dizziness
- increased drowsiness or confusion
- clumsiness
- greater than normal hunger
- excessive sweating
- increased irritability and nervousness
If you notice any of the above symptoms or are unsure, consult your doctor immediately. Your doctor may need to change the treatment.
Among the symptoms of hyperglycemiaare:
- thirst or hunger
- need to urinate more frequently
- drowsiness
- general discomfort
- blurred vision
- chest or back pain
- shortness of breath
Signs of High Fat Levels
Among the symptoms of high fat levelsare:
- chest pain
- pain under the ribs similar to heartburn or indigestion
- general discomfort
Tell your doctor if you notice any of the above adverse effects.
Reporting Adverse Effects
If you experience any adverse effect, consult your doctor, pharmacist, or nurse, even if it is a possible adverse effect not listed in this leaflet. You can also report them directly through the national reporting system included in Appendix V. By reporting adverse effects, you can contribute to providing more information on the safety of this medicine.
5. Storage of Myalepta
Keep this medicine out of sight and reach of children.
Do not use this medicine after the expiration date stated on the packaging after CAD and on the vial after EXP. The expiration date is the last day of the month indicated.
Store in a refrigerator (between 2°C and 8°C). Keep the vial in the outer packaging to protect it from light.
After reconstitution, the solution must be administered immediately and cannot be stored for later use. Discard unused medicine.
Do not use this medicine if you notice that the solution is not transparent, has color, or contains particles.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of packaging and medicines that are no longer needed. This will help protect the environment.
6. Container Contents and Additional Information
Myalepta Composition
- The active ingredient is metreleptin.
Each vial contains 5.8 milligrams of metreleptin. After diluting the vial contents in 1.1 milliliters of water for injectable preparations, each milliliter will contain 5 milligrams of metreleptin.
- The other components are: glycine, sucrose, polysorbate 20, glutamic acid, and sodium hydroxide (for pH adjustment).
Appearance of Myalepta and Container Contents
Myalepta is presented as a powder for injectable solution. It is a white powder supplied in a glass vial with a rubber stopper and an aluminum seal with a easy-open plastic blue cap.
Myalepta is available in packs containing 1 or 30 vials.
Only some pack sizes may be marketed in your country.
Your doctor, nurse, or pharmacist will provide you with the appropriate syringes and needles, swabs, and water for injectable preparations to allow you to prepare and inject Myalepta. They will also provide you with a container for disposing of sharp objects so that you can safely dispose of the used vials, syringes, and needles.
Marketing Authorization Holder
Chiesi Farmaceutici S.p.A.
Via Palermo 26/A
43122 Parma
Italy
Manufacturer
Amryt Pharmaceuticals DAC
45 Mespil Road
Dublin 4
Ireland
You can request more information about this medicine by contacting the local representative of the marketing authorization holder:
België/Belgique/Belgien Chiesi sa/nv Tél/Tel: + 32 (0)2 788 42 00 | Lietuva ExCEEd Orphan s.r.o. Bucharova 2657/12, Prague 5, 158 00 Czech Republic Tel.: +370 661 663 99 |
| Luxembourg/Luxemburg Chiesi sa/nv Tél/Tel: + 32 (0)2 788 42 00 |
Ceská republika ExCEEd Orphan s.r.o. Bucharova 2657/12, Prague 5, 158 00 Czech Republic Tel: +420 724 321 774 [email protected] | Magyarország ExCEEd Orphan s.r.o. Bucharova 2657/12, Prague 5, 158 00 Czech Republic Tel.: +36 20 399 4269 |
Danmark Chiesi Pharma AB Tlf.: + 46 8 753 35 20 | Malta Amryt Pharmaceuticals DAC Tel: +44 1604 549952 |
Deutschland Chiesi GmbH Tel: + 49 40 89724-0 | Nederland Chiesi Pharmaceuticals B.V. Tel: + 31 88 501 64 00 |
Eesti ExCEEd Orphan s.r.o. Bucharova 2657/12, Prague 5, 158 00 Czech Republic Tel.: +370 661 663 99 | Norge Chiesi Pharma AB Tlf: + 46 8 753 35 20 |
Ελλ?δα Amryt Pharmaceuticals DAC Tηλ: +800 44 474447 Tηλ: +44 1604 549952 | Österreich Chiesi Pharmaceuticals GmbH Tel: + 43 1 4073919 |
España Chiesi España, S.A.U. Tel: + 34 93 494 8000 | Polska ExCEEd Orphan s.r.o. Bucharova 2657/12, Prague 5, 158 00 Czech Republic Tel.: +48 502 188 023 |
France Chiesi S.A.S. Tél: + 33 1 47688899 | Portugal Chiesi Farmaceutici S.p.A. Tel: + 39 0521 2791 |
Hrvatska ExCEEd Orphan Distribution d.o.o. Savska cesta 32, Zagreb, 100 00 Croatia Tel: +385 99 320 0330 | România ExCEEd Orphan s.r.o. Bucharova 2657/12, Prague 5, 158 00 Czech Republic Tel: +40 744 366 015 |
Ireland Chiesi Farmaceutici S.p.A. Tel: + 39 0521 2791 | Slovenija ExCEEd Orphan s.r.o. Bucharova 2657/12, Prague 5, 158 00 Czech Republic Tel: +386 30 210 050 |
Ísland Chiesi Pharma AB Sími: +46 8 753 35 20 | Slovenská republika ExCEEd Orphan s.r.o. Bucharova 2657/12, Prague 5, 158 00 Czech Republic Tel: +420 608 076 274 |
Italia Chiesi Italia S.p.A. Tel: + 39 0521 2791 | Suomi/Finland Chiesi Pharma AB Puh/Tel: +46 8 753 35 20 |
Κ?προς Amryt Pharmaceuticals DAC Tηλ: +800 44 474447 Tηλ: +44 1604 549952 | Sverige Chiesi Pharma AB Tel: +46 8 753 35 20 |
Latvija ExCEEd Orphan s.r.o. Bucharova 2657/12, Prague 5, 158 00 Czech Republic Tel.: +370 661 663 99 |
Date of Last Revision of this Leaflet: June 2025
This medicinal product has been authorized under “exceptional circumstances”. This means that due to the rarity of this disease, it has not been possible to obtain complete information on this medicinal product.
The European Medicines Agency will review any new information on this medicinal product that may become available every year and this leaflet will be updated as necessary.
Other Sources of Information
Detailed information on this medicinal product is available on the European Medicines Agency website: https://www.ema.europa.eu, and on the website of the Spanish Agency for Medicines and Health Products (AEMPS) (https://www.aemps.gob.es/).
There are also links to other websites about rare diseases and orphan medicines.
Instructions for Use
Before using Myalepta, you must first read sections 1-6 of this leaflet and then the Instructions for Use.
Before starting self-administration of this medicine at home, your doctor, nurse, or pharmacist will show you how to prepare and inject Myalepta. Contact them if you have any questions or need more information or help. Take the time to prepare and inject your medicine carefully; this includes the time it takes for the vial to reach room temperature after removing it from the refrigerator, which can take approximately 20 minutes.
Additional Training Information
You can find videos and additional training information that will help you understand how to use Myalepta correctly. Your doctor will show you how to access this information.
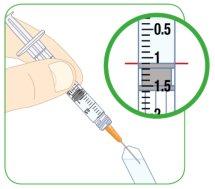
Syringe Reading
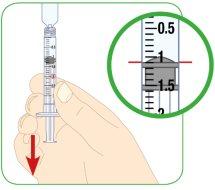
Align the top edge of the plunger with the line corresponding to the prescribed dose. Then, you can find an example of the different syringe sizes. If the syringe is not the same or has different dose marks, consult your doctor, nurse, or pharmacist for more information.
Using the 0.3 ml Syringe
- The 0.3 ml syringe shows the injection amount in “U”, instead of “ml”.
- “U” means “units”.
- 1 U is equivalent to 0.01 ml.
- The 5 U intervals are shown with a number and a large line. This is equivalent to 0.05 ml.
- The U change is shown with a smaller line between the large lines. This is equivalent to 0.01 ml.
- The 0.5 U change is shown with a small line between the 1 U lines. This is equivalent to 0.005 ml.
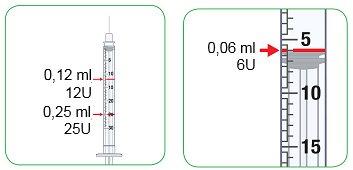
- The last column of the following table shows the “unit” measurement of the syringe for the different possible doses of the medicine prescribed by your doctor, nurse, or pharmacist, and will help you inject the Myalepta solution using the small 0.3 ml syringe.
Converting Dose from “ml” to “units” with the 0.3 ml Syringe
Child's Weight | Myalepta Dose | Amount of Myalepta Solution Mixed | Amount of Myalepta Solution to be Injected in “units” with the 0.3 ml Syringe |
9 kg | 0.54 mg | 0.10 ml | 10 |
10 kg | 0.60 mg | 0.12 ml | 12 |
11 kg | 0.66 mg | 0.13 ml | 13 |
12 kg | 0.72 mg | 0.14 ml | 14 |
13 kg | 0.78 mg | 0.15 ml | 15 |
14 kg | 0.84 mg | 0.16 ml | 16 |
15 kg | 0.90 mg | 0.18 ml | 18 |
16 kg | 0.96 mg | 0.19 ml | 19 |
17 kg | 1.02 mg | 0.20 ml | 20 |
18 kg | 1.08 mg | 0.21 ml | 21 |
19 kg | 1.14 mg | 0.22 ml | 22 |
20 kg | 1.20 mg | 0.24 ml | 24 |
21 kg | 1.26 mg | 0.25 ml | 25 |
22 kg | 1.32 mg | 0.26 ml | 26 |
23 kg | 1.38 mg | 0.27 ml | 27 |
24 kg | 1.44 mg | 0.28 ml | 28 |
25 kg | 1.50 mg | 0.30 ml | 30 |
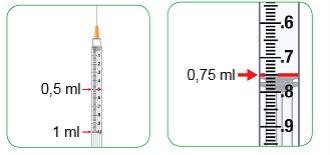
Using the 1 ml Syringe
- This syringe shows the injection amount in ml so you can inject the amount indicated by your doctor, nurse, or pharmacist. You do not need to convert the amount of ml to units.
- You will be provided with the 1 ml syringe if the daily dose is more than 1.5 mg and up to 5 mg, which is between 0.3 and 1.0 ml of Myalepta solution.
- The 0.1 ml intervals are shown with a number and a large line.
- The 0.05 ml intervals are shown with a medium-sized line.
- The 0.01 ml intervals are shown with a smaller line.
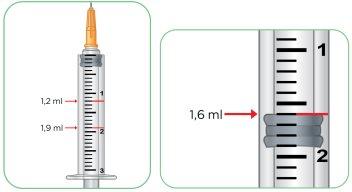
Using the 3.0 ml Syringe
- This syringe shows the injection amount in ml so you can inject the amount indicated by your doctor, nurse, or pharmacist. You do not need to convert the amount of ml to units.
- You will be provided with the 3.0 ml syringe if the daily dose is more than 5 mg and up to 10 mg, which is more than 1.0 ml of Myalepta solution.
- The 0.5 ml intervals are shown with a number next to a large line.
- The 0.1 ml intervals are shown with a smaller line between the large lines.
Step A: Preparation
- Gather all the materials you will need for the injection. These will be provided by your doctor, nurse, or pharmacist.
Place the following items on a white, well-lit work surface:
- a glass vial of Myalepta powder
- a container with water for injectable preparations to dilute the Myalepta powder
- Water for injectable preparations may be supplied in plastic or glass ampoules, or in glass vials with a rubber stopper.
- alcohol swabs (to clean the skin at the injection site and to clean the top of the vials)
- a container for disposing of sharp objects (to safely dispose of the injection materials after use)
You will also need two syringes:
- a 3 ml syringe and a 21-gauge, 40 mm needle for diluting the powder
- a syringe for injection with a much shorter needle for injecting the solution under the skin
Your doctor, nurse, or pharmacist will choose the size of this syringe that you will use for the Myalepta dose.
- If the dose is 1.5 mg or less, you will use a 0.3 ml syringe.
- If the dose is more than 1.5 mg and up to 5 mg, you will use a 1 ml syringe.
- If the dose is more than 5 mg, you will use a 3.0 ml syringe.
- If the dose is more than 5 mg, your doctor, nurse, or pharmacist may instruct you to inject the dose in two separate injections. See section 3 “Amount to be Injected” for more information.
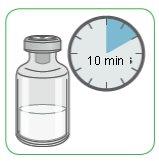
- Before preparing the Myalepta solution, let the powder vial reach room temperature for about 10 minutes.
- Wash your hands before preparing the medicine.
Step B: Filling the 3 ml Syringe with 1.1 ml of Water for Injectable Preparations
- Remove the 3 ml syringe from the plastic wrapper. Always use a new syringe.
- The needle and 3 ml syringe will be provided separately.
- The way you connect the needle to the syringe will depend on whether the water for injectable preparations is provided in a plastic ampoule, a glass ampoule, or a glass vial (follow the specific instructions below).
- Draw up 1.1 ml of water for injectable preparations into a 3 ml syringe.
Your doctor, nurse, or pharmacist will provide you with “water for injectable preparations” with the syringes and the medicine vial. This must be mixed with the Myalepta powder to dissolve it and obtain the liquid medicine that you must inject. The water for injectable preparations will be provided in:
- a plastic ampoule;
- a glass ampoule; or
- a glass vial (with a rubber stopper)
Always use a new ampoule, water for injectable preparations, or vial. Never use leftover water for injectable preparations from a previous Myalepta solution preparation.
Plastic Ampoule of Water for Injectable Preparations
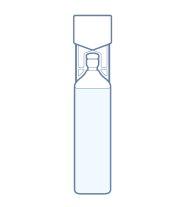
The plastic ampoule is a container with a snap-off top.
To draw up the water for injectable preparations, snap off the top of the ampoule.
- Hold the ampoule with the top facing up.
- Hold the ampoule by the bottom with one hand and the top with the other hand.
- Without moving the bottom of the ampoule, carefully snap off the top.
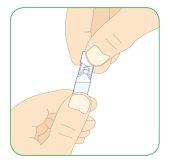
- Do not attach the needle to the syringe.
- Without attaching the needle, insert the tip of the 3 ml syringe as far as possible into the top of the plastic ampoule.
Keep the syringe inside the ampoule and turn it upside down. The syringe should now be facing up.
Keep the syringe inside the ampoule and carefully pull back the plunger.
- Pull back the plunger until the top edge of the plunger is aligned with the black mark at 1.1 ml.
- You must check that there are no air pockets or air bubbles in the 3 ml syringe. See steps 6 to 8 below for instructions on removing air pockets or air bubbles from the syringe.
- Remove the syringe from the plastic ampoule.
Attach the needle to the syringe.
- Do not overtighten the needle.
- Do not remove the needle protector.
- Do not touch the needle.
Glass Ampoule of Water for Injectable Preparations
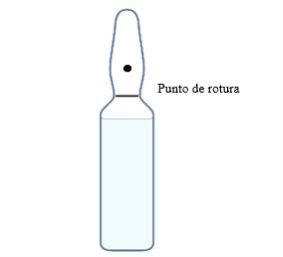
The glass ampoule is a sealed container.
Before opening the glass ampoule of water for injectable preparations, prepare the 3 ml syringe by attaching the needle. Do not overtighten the needle.
- Remove the needle protector.
- Do not touch the needle.
To draw up the water for injectable preparations, open the ampoule by breaking it at the break point, as shown in the image.
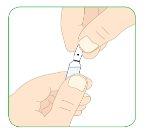
- Hold the ampoule with the tip facing up.
- Clean the break point of the ampoule with the alcohol swab.
- Hold the ampoule by the bottom with one hand and the top with the other hand.
- Without moving the bottom of the ampoule, break the top.
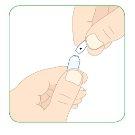
Maintain the needle inside the ampoule and carefully pull the plunger.
- Pull the plunger until the top edge of it aligns with the 1.1 ml black mark.
- You must check that there are no air pockets or bubbles in the 3 ml syringe. Refer to steps 6 to 8 below on removing air pockets or bubbles from the syringe.
Glass vial of water for injectable preparations
The glass vial will have a plastic cap that you should remove, and underneath, you will find a rubber seal.
- Do not remove the rubber seal.
Connect the needle to the 3 ml syringe. Do not tighten the needle too much.
- Remove the needle protector.
- Do not touch the needle.
- Pull the plunger until you reach the 1.1 ml mark to introduce air into the syringe.
Place the vial on a rigid and flat surface.
- Insert the needle of the 3 ml syringe into the vial through the rubber seal.
- The needle should be facing downwards.
- The needle should be inserted completely into the vial.
Push the plunger fully.
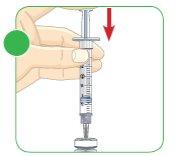
Maintain the needle inside the vial and turn it around. The needle should now be facing upwards.
- Do not extract the needle from the vial.
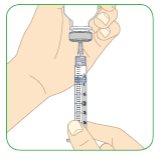
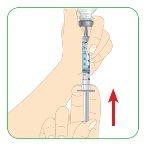
Carefully pull the plunger.
- Pull the plunger until the top edge of it aligns with the 1.1 ml black mark.
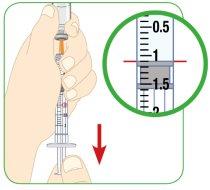
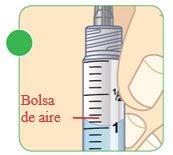
- Regardless of whether you have extracted the water for injectable preparations from a vial or an ampoule, you must check that there are no air pockets or bubbles in the 3 ml syringe.
- Sometimes large air masses (air pockets) become trapped inside the syringe. You may also see smaller air bubbles inside the syringe.
- You must extract the air pockets or bubbles from the syringeto ensure that it contains the correct amount of sterile water.
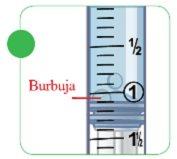
- Extract the air pockets and bubbles.
With the glass vial or plastic ampoule
- With the syringe still inserted in the glass vial or plastic ampoule, tap the side of the syringe to displace the air pocket or bubbles towards the top of the syringe.
- Carefully press the plunger to make the air exit the syringe.
With the glass ampoule
- Extract the syringe from the ampoule and hold it with the needle facing upwards.
- Tap the side of the syringe to displace the air pocket or bubbles towards the top of the syringe.
- Carefully press the plunger to make the air exit the syringe.
- Check the amount of water for injectable preparations
- If the syringe contains less than 1.1 ml of water for injectable preparations, extract more water for injectable preparations into the syringe and repeat steps 6 and 7 until the syringe contains 1.1 ml.
- When the syringe contains 1.1 ml of water for injectable preparations, remove it from the vial or ampoule.
- Do not move the plunger.
- Do not touch the exposed needle of the syringe, as it is sterilized and could damage the needle or cause injury.
Step C: Myalepta dilution
- Make sure the Myalepta powder vial has been out of the refrigerator for at least 10 minutes to reach room temperature.
- Remove the plastic cap from the Myalepta powder vial.
- Place the vial on a rigid and flat surface.
- Clean the top of the vial with a cotton swab.
- Insert the needle of the 3 ml syringe with 1.1 ml of water for injectable preparations completely into the Myalepta vial containing the powder.
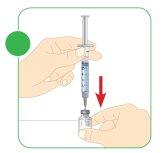
- Hold the vial at a 45° angle to the table and slowly press the plunger with your thumb until the end.
- The water for injectable preparations should slide down the inner wall of the vial.
- You must inject all the water into the vial.
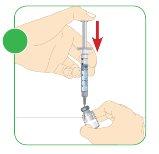
- Remove the needle from the vial and discard the syringe in the container for disposing of sharp objects.
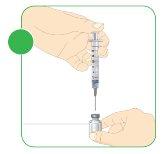
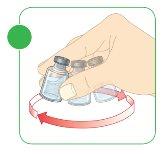
- Mix the powder and the water for injectable preparations.
- Gently rotate the vial with circular movements.
- Continue until the powder dissolves and the liquid is transparent. Do not shake or mix forcefully.
- The solution will become transparent in less than five minutes.
When well mixed, the Myalepta solution should be transparent and not contain any dry powder residue, bubbles, or foam. Do not use the solution if it is not transparent or contains particles. Discard it and start again from step 1.
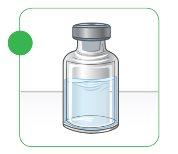
Step D: filling the syringe with injectable Myalepta
- To inject the Myalepta solution, you will use a new injection syringe that will be 0.3 ml, 1.0 ml, or 3.0 ml, which your doctor, nurse, or pharmacist will have provided. Remove the needle protector.
- Do nottouch the needle.
- Do notmove the plunger.
- Insert the needle completely into the vial containing the dissolved Myalepta solution through the central part of the rubber stopper.
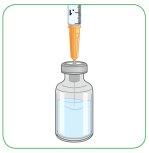
- Keep the needle inside the vial and turn it around.
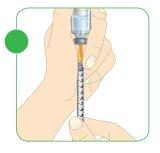
- Keep the needle inside the vial and pull the plunger.
- The top edge of the plunger should align with the black mark on the syringe that corresponds to the amount of Myalepta solution to be injected.
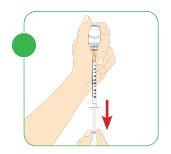
- Check that there are no air pockets or bubbles.
- If you see an air pocket or bubbles, follow the same instructionsdescribed in step 7 to remove air from the syringe.
- If the syringe contains the correct dose of Myalepta for you, remove the needle from the vial.
- Do notmove the plunger.
- Do nottouch the needle.
Step E: selection and preparation of the injection site
- Choose the place where you want to inject Myalepta carefully. You can inject this medication in the following areas:
- stomach area (abdomen), except for the area 5 cm around the navel
- thigh
- upper back of the arm
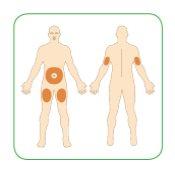
If you want to perform all injections in the same area of the body, do not use the same point where you performed the previous injection.
- If you are injecting other medications, do not inject Myalepta in the same place you used for the other medications.
- Clean the area where the injection will be performed with a clean alcohol swab and let the skin dry.
- Do not touch the cleaned area until you are injecting Myalepta.
Step F: Myalepta injection
Important:Myalepta should be injected under the skin (“subcutaneously”). Do notinject into a muscle.
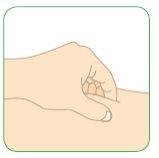
- To perform the injection under the skin, pinch the skin with one hand at the place where you will inject.
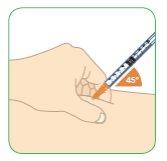
- With the other hand, hold the syringe as if it were a pencil.
- Insert the needle gently into the skin at an angle of approximately 45° with respect to the body.
- Do notinsert the needle into a muscle.
- The needle is short and should be inserted into the skin completely at a 45° angle.
- Use your thumb to gently push the plunger until the end.
- Inject all the medication.
- If there is medication left in the syringe, the full dose has not been administered.
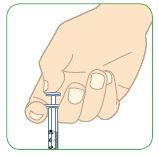
- Remove the syringe from the skin.
Step G: disposal of used materials
- Immediately discard the two used syringes and all the caps, vials, or ampoules in the container for disposing of sharp objects.
- Talk to your doctor, nurse, or pharmacist about how to properly dispose of your container for sharp objects when it is full. There may be local regulations for this.
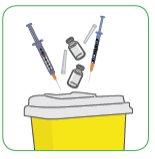
Important
- Do not use syringes more than once. Always use new syringes.
- The vials may be almost full with the product after extracting the required dose. The remaining solution should be discarded after use.
- Do not dissolve another dose of Myalepta powder using a vial or ampoule that contains unused water for injectable preparations. You must discard the unused water for injectable preparations in your container for sharp objects. Always use a new vial or ampoule of water for injectable preparations each time you prepare the Myalepta powder dilution.
- Do not recycle syringes, caps, or the container for disposing of sharp objects, nor dispose of them in household waste.
- Keep the container for disposing of sharp objects out of the reach of children at all times.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to MYALEPTA 5.8 mg POWDER FOR INJECTABLE SOLUTIONDosage form: INJECTABLE, 11.3 mgActive substance: metreleptinManufacturer: Chiesi Farmaceutici S.P.A.Prescription requiredDosage form: INJECTABLE, 3.0 mgActive substance: metreleptinManufacturer: Chiesi Farmaceutici S.P.A.Prescription requiredDosage form: TABLET, 200 mgActive substance: carglumic acidManufacturer: Laboratorios Tillomed Spain S.L.UPrescription required
Online doctors for MYALEPTA 5.8 mg POWDER FOR INJECTABLE SOLUTION
Discuss questions about MYALEPTA 5.8 mg POWDER FOR INJECTABLE SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions
















