
MOUNJARO 15 mg/dose KwikPen prefilled pen injector solution

How to use MOUNJARO 15 mg/dose KwikPen prefilled pen injector solution
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient |
Mounjaro 2.5mg/dose KwikPen injectable solution in a pre-filled pen Mounjaro 5mg/dose KwikPen injectable solution in a pre-filled pen Mounjaro 7.5mg/dose KwikPen injectable solution in a pre-filled pen Mounjaro 10mg/dose KwikPen injectable solution in a pre-filled pen Mounjaro 12.5mg/dose KwikPen injectable solution in a pre-filled pen Mounjaro 15mg/dose KwikPen injectable solution in a pre-filled pen |
tirzepatida
This medicine is subject to additional monitoring, which will help to quickly identify new safety information. You can contribute by reporting any side effects you may have. The last part of section 4 will tell you how to report side effects.
Read all of this leaflet carefully before you start using this medicine, because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, nurse, or pharmacist.
- This medicine has been prescribed for you only, and you should not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, nurse, or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What Mounjaro KwikPen is and what it is used for
- What you need to know before you use Mounjaro KwikPen
- How to use Mounjaro KwikPen
- Possible side effects
- Storage of Mounjaro KwikPen
- Contents of the pack and other information
1. What Mounjaro is and what it is used for
Mounjaro contains the active substance tirzepatida and is used to treat adults with type 2 diabetes mellitus. Mounjaro lowers blood sugar levels only when blood sugar levels are high.
Mounjaro is also used to treat adults with obesity or overweight (with a BMI of at least 27 kg/m2). Mounjaro affects appetite regulation, which can help you eat less food and reduce your body weight.
In type 2 diabetes, Mounjaro is used:
- alone when you cannot take metformin (another diabetes medicine).
- or in combination with other diabetes medicines when these are not enough to control the sugar levels in your blood. These other medicines may be taken orally and/or may be an insulin injection.
Mounjaro is also used together with diet and exercise to lose weight and help maintain weight under control in adults who have:
- a BMI of 30 kg/m² or more (obesity) or
- a BMI of at least 27 kg/m², but less than 30 kg/m² (overweight) and weight-related health problems (such as prediabetes, type 2 diabetes, high blood pressure, abnormal blood fat levels, breathing problems during sleep called "obstructive sleep apnea" or a history of heart attack, stroke, or vascular problems)
Body Mass Index (BMI) is a measure of your weight in relation to your height.
In patients with obstructive sleep apnea (OSA) and obesity, Mounjaro can be used with or without positive airway pressure therapy.
It is important that you follow the dietary and physical activity advice given by your doctor, nurse, or pharmacist.
2. What you need to know before you use Mounjaro KwikPen
Do not use Mounjaro KwikPen
- if you are allergic to tirzepatida or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Talk to your doctor, nurse, or pharmacist before starting Mounjaro if:
- you have severe problems with food digestion or food stays in your stomach for longer than usual (including severe gastroparesis).
- you have ever had pancreatitis (inflammation of the pancreas, which can cause severe stomach and back pain that does not go away).
- you have any eye problems (diabetic retinopathy or macular edema).
- you are taking a sulfonylurea (another diabetes medicine) or insulin for diabetes, as low blood sugar (hypoglycemia) may occur. Your doctor may need to adjust the dose of these medicines to reduce this risk.
When starting treatment with Mounjaro, you may experience fluid loss/dehydration, such as vomiting, nausea, and/or diarrhea, which can cause a decrease in kidney function. To avoid dehydration, it is essential to drink plenty of fluids. Contact your doctor if you have any questions or concerns.
If you know you are going to have surgery, inform your doctor that you are taking Mounjaro.
Children and adolescents
This medicine must not be used in children and adolescents under 18 years of age because it has not been studied in this age group.
Other medicines and Mounjaro
Tell your doctor, nurse, or pharmacist if you are using, have recently used, or might use any other medicines.
Pregnancy
If you are pregnant, think you may be pregnant, or plan to become pregnant, consult your doctor before using this medicine. This medicine should not be used during pregnancy, as its effects on the fetus are unknown. Therefore, contraceptive methods are recommended while using this medicine.
Breast-feeding
It is not known whether tirzepatida passes into breast milk. A risk to newborns/babies cannot be ruled out. If you are breast-feeding or plan to breast-feed, consult your doctor before using this medicine. You and your doctor must decide whether to stop breast-feeding or stop using Mounjaro.
Driving and using machines
This medicine is unlikely to affect your ability to drive or use machines. However, if you use Mounjaro with a sulfonylurea or insulin, low blood sugar (hypoglycemia) may occur, which can reduce your ability to concentrate. Avoid driving or using machines if you have any signs of low blood sugar, such as headache, drowsiness, weakness, dizziness, hunger, confusion, irritability, rapid heartbeat, and sweating (see section 4). See section 2, "Warnings and precautions" for information on the increased risk of low blood sugar. Consult your doctor for more information.
Mounjaro KwikPen contains sodium
This medicine contains less than 1 mmol of sodium (23 mg) per dose; this is essentially "sodium-free".
Mounjaro KwikPen contains benzyl alcohol
This medicine contains 5.4 mg of benzyl alcohol in each 0.6 ml dose. Benzyl alcohol may cause allergic reactions.
Consult your doctor, nurse, or pharmacist if you are pregnant, breast-feeding, or have liver or kidney disease. This is because large amounts of benzyl alcohol can accumulate in the body and cause side effects (so-called "metabolic acidosis").
3. How to use Mounjaro KwikPen
Follow the instructions for administration of this medicine exactly as told by your doctor or pharmacist. If you are unsure, consult your doctor or pharmacist again.
A small amount of medicine may remain in the pen after you have correctly administered all doses. Do not attempt to use the leftover medicine. After four doses, the pen should be disposed of properly.
How much to use
- The initial dose is 2.5 mg once a week for four weeks. After four weeks, your doctor will increase your dose to 5 mg once a week.
- Your doctor may increase your dose in increments of 2.5 mg up to 7.5 mg, 10 mg, 12.5 mg, or 15 mg once a week if needed. In each case, your doctor will tell you to stay on a specific dose for at least 4 weeks before moving to a higher dose.
Do not change your dose unless your doctor has told you to.
When to use Mounjaro
You can use your pen at any time of day, with or without food. If possible, you should use it on the same day each week. To help you remember when to use Mounjaro, you can write it down in a calendar if you wish.
If necessary, you can change the day of your weekly Mounjaro injection, provided that at least 3 days have passed since your last injection. After selecting a new dosing day, continue with once-weekly administration on that new day.
How to inject Mounjaro KwikPen
Mounjaro is injected under the skin (subcutaneous injection) in the abdominal area at least 5 cm away from the navel or in the upper thigh or upper arm. You may need help from another person if you want to inject into the upper arm.
If you wish, you can inject into the same area of your body each week. However, make sure to choose different injection sites within that area. If you also inject insulin, choose a different injection site for that injection.
Before using Mounjaro KwikPen, carefully read the "Instructions for use" of the pen.
Monitoring blood sugar levels
If you are using Mounjaro with a sulfonylurea or insulin, it is essential to monitor your blood sugar levels as instructed by your doctor, nurse, or pharmacist (see section 2, "Warnings and precautions").
If you use more Mounjaro than you should
If you use more Mounjaro than you should, consult your doctor immediately. Too much medicine can cause your blood sugar levels to drop too low (hypoglycemia) and may also cause nausea or vomiting.
If you forget to use Mounjaro
If you forget to inject a dose and,
- it has been 4days or less since you should have used Mounjaro, use it as soon as you remember. Then inject your next dose as usual on your scheduled day.
- it has been more than 4days since you should have used Mounjaro, skip the missed dose. Then inject your next dose as usual on your scheduled day.
Do not inject a double dose to make up for a missed dose. The minimum time between two doses must be at least 3 days.
If you stop using Mounjaro
Do not stop using Mounjaro without consulting your doctor. If you stop using Mounjaro and have type 2 diabetes, your blood sugar levels may increase.
If you have any other questions about using this medicine, ask your doctor, nurse, or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Serious side effects
Uncommon(may affect up to 1 in 100 people)
- Pancreatitis (inflammation of the pancreas) that could cause severe stomach and back pain that does not go away. See your doctor immediately if you experience these symptoms.
Rare(may affect up to 1 in 1,000 people)
- Severe allergic reactions (e.g., anaphylactic reaction, angioedema). You should get medical help immediately and inform your doctor if you experience symptoms such as breathing problems, rapid swelling of the lips, tongue, and/or throat with difficulty swallowing, and rapid heartbeat.
Other side effects
Very common(may affect more than 1 in 10 people)
- Nausea
- Diarrhea
- Stomach pain (abdominal pain) reported in patients treated for weight control
- Vomiting reported in patients treated for weight control
- Constipation reported in patients treated for weight control
These side effects are usually not serious. Nausea, diarrhea, and vomiting are more common when starting tirzepatida but decrease over time in most patients.
- Low blood sugar (hypoglycemia) is very common when tirzepatida is used with a sulfonylurea and/or insulin. If you are taking a sulfonylurea or insulin for type 2 diabetes, you may need to have your dose reduced while using tirzepatida (see section 2, "Warnings and precautions"). Symptoms of low blood sugar can include headache, drowsiness, weakness, dizziness, hunger, confusion, irritability, rapid heartbeat, and sweating. Your doctor should tell you how to treat low blood sugar levels.
Common(may affect up to 1 in 10 people)
- Low blood sugar (hypoglycemia) when tirzepatida is used for type 2 diabetes with metformin and a sodium-glucose cotransporter 2 inhibitor (another diabetes medicine)
- Allergic reaction (hypersensitivity) (e.g., rash, itching, and eczema)
- Dizziness reported in patients treated for weight control
- Low blood pressure reported in patients treated for weight control
- Decreased appetite reported in patients treated for type 2 diabetes
- Stomach pain (abdominal pain) reported in patients treated for type 2 diabetes
- Vomiting reported in patients treated for type 2 diabetes – usually decreases over time
- Indigestion (dyspepsia)
- Constipation reported in patients treated for type 2 diabetes
- Bloating
- Belching
- Gas (flatulence)
- Acid reflux or heartburn (also called gastroesophageal reflux disease – GERD) – a disease caused by stomach acid flowing back up into the tube that connects the stomach to the mouth
- Hair loss reported in patients treated for weight control
- Fatigue
- Injection site reactions (e.g., itching or redness)
- Rapid heartbeat
- Increased levels of pancreatic enzymes (such as lipase and amylase) in the blood.
- Increased levels of calcitonin in the blood in patients treated for weight control.
Uncommon(may affect up to 1 in 100 people)
- Low blood sugar (hypoglycemia) when tirzepatida is used with metformin for type 2 diabetes
- Gallstones
- Gallbladder inflammation
- Weight loss reported in patients treated for type 2 diabetes
- Pain at the injection site
- Increased levels of calcitonin in the blood in patients treated for type 2 diabetes or for OSA with obesity
- Altered sense of taste
- Changes in skin sensitivity
- Delayed stomach emptying
Reporting of side effects
If you experience any side effects, talk to your doctor, nurse, or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly through the Spanish Pharmacovigilance System for Human Use Medicines: www.notificaRAM.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Mounjaro KwikPen
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the label of the pen and on the carton after EXP. The expiry date is the last day of the month shown.
Store in a refrigerator (2°C to 8°C). Do not freeze. If the pen has been frozen, DO NOT USE IT.
Mounjaro KwikPen can be stored without refrigeration below 30°C for a maximum of 30 days after first use, and then the pen must be discarded.
Do not use this medicine if you notice that the pen is damaged or the medicine is cloudy, has color, or contains particles.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Container Contents and Additional Information
Composition of Mounjaro KwikPen
The active ingredient is tirzepatide.
Mounjaro 2.5mg/doseKwikPen:Each dose contains 2.5 mg of tirzepatide in 0.6 ml of solution. Each pre-filled multidose pen contains 10 mg of tirzepatide in 2.4 ml (4.17 mg/ml). Each pen delivers 4 doses of 2.5 mg.
Mounjaro 5mg/doseKwikPen:Each dose contains 5 mg of tirzepatide in 0.6 ml of solution. Each pre-filled multidose pen contains 20 mg of tirzepatide in 2.4 ml (8.33 mg/ml). Each pen delivers 4 doses of 5 mg.
Mounjaro 7.5mg/doseKwikPen:Each dose contains 7.5 mg of tirzepatide in 0.6 ml of solution. Each pre-filled multidose pen contains 30 mg of tirzepatide in 2.4 ml (12.5 mg/ml). Each pen delivers 4 doses of 7.5 mg.
Mounjaro 10mg/doseKwikPen:Each dose contains 10 mg of tirzepatide in 0.6 ml of solution. Each pre-filled multidose pen contains 40 mg of tirzepatide in 2.4 ml (16.7 mg/ml). Each pen delivers 4 doses of 10 mg.
Mounjaro 12.5mg/doseKwikPen:Each dose contains 12.5 mg of tirzepatide in 0.6 ml of solution. Each pre-filled multidose pen contains 50 mg of tirzepatide in 2.4 ml (20.8 mg/ml). Each pen delivers 4 doses of 12.5 mg.
Mounjaro 15mg/doseKwikPen:Each dose contains 15 mg of tirzepatide in 0.6 ml of solution. Each pre-filled multidose pen contains 60 mg of tirzepatide in 2.4 ml (25 mg/ml). Each pen delivers 4 doses of 15 mg.
The other components are disodium hydrogen phosphate heptahydrate (E339), benzyl alcohol (E1519) (see section 2 "Mounjaro KwikPen contains benzyl alcohol" for more information), glycerol, phenol, sodium chloride, and sodium hydroxide (see section 2 "Mounjaro contains sodium" for more information); concentrated hydrochloric acid and water for injectable preparations.
Appearance of the Product and Container Contents
Mounjaro is a clear, colorless to slightly yellowish injectable solution in a pre-filled pen (KwikPen).
Each KwikPen contains 2.4 ml of injectable solution (4 doses of 0.6 ml) and excess for purging.
Needles are not included.
Package sizes of 1 and 3 KwikPens.
Only some package sizes may be marketed.
Marketing Authorization Holder
Eli Lilly Nederland B.V., Papendorpseweg 83, 3528 BJ Utrecht, Netherlands.
Manufacturer
Eli Lilly Italia S.p.A., Via Gramsci 731/733, 50019, Sesto Fiorentino, Florence (FI), Italy
Lilly S.A., Avda. de la Industria, 30, 28108 Alcobendas, Madrid, Spain
Lilly France, 2, rue du Colonel Lilly, 67640 Fegersheim, France
Millmount Healthcare Limited, Block 7 City North Business Campus, Stamullen, K32 YD60, Ireland
Millmount Healthcare Limited, IDA Science And Technology Park, Mullagharlin, Dundalk, Co. Louth, A91 DET0, Ireland
You can request more information about this medicine by contacting the local representative of the marketing authorization holder:
Belgium/België/Belgien Eli Lilly Benelux S.A./N.V. Tel: + 32-(0)2 548 84 84 | Lithuania Eli Lilly Lietuva Tel: +370 (5) 2649600 |
Bulgaria Eli Lilly Bulgaria EOOD Tel: + 359 2 491 41 40 | Luxembourg/Luxemburg Eli Lilly Benelux S.A./N.V. Tel: + 32-(0)2 548 84 84 |
Czech Republic ELI LILLY CR, s.r.o. Tel: + 420 234 664 111 | Hungary Lilly Hungária Kft. Tel: + 36 1 328 5100 |
Denmark Eli Lilly Danmark A/S Tel: +45 45 26 60 00 | Malta Charles de Giorgio Ltd. Tel: + 356 25600 500 |
Germany Lilly Deutschland GmbH Tel: + 49-(0) 6172 273 2222 | Netherlands Eli Lilly Nederland B.V. Tel: + 31-(0) 30 60 25 800 |
Estonia Eli Lilly Nederland B.V. Tel: +372 6 817 280 | Norway Eli Lilly Norge A.S. Tel: + 47 22 88 18 00 |
Greece ΦΑΡΜΑΣΕΡΒ-ΛΙΛΛΥ Α.Ε.Β.Ε. Tel: +30 210 629 4600 | Austria Eli Lilly Ges.m.b.H. Tel: + 43-(0) 1 20609 1270 |
Spain Lilly S.A. Tel: + 34-91 663 50 00 | Poland Eli Lilly Polska Sp. z o.o. Tel: +48 22 440 33 00 |
France Lilly France Tel: +33-(0) 1 55 49 34 34 | Portugal Lilly Portugal Produtos Farmacêuticos, Lda Tel: + 351-21-4126600 |
Croatia Eli Lilly Hrvatska d.o.o. Tel: +385 1 2350 999 | Romania Eli Lilly România S.R.L. Tel: + 40 21 4023000 |
Ireland Eli Lilly and Company (Ireland) Limited Tel: + 353-(0) 1 661 4377 | Slovenia Eli Lilly farmacevtska družba, d.o.o. Tel: +386 (0)1 580 00 10 |
Iceland Icepharma hf. Tel: + 354 540 8000 | Slovakia Eli Lilly Slovakia s.r.o. Tel: + 421 220 663 111 |
Italy Eli Lilly Italia S.p.A. Tel: + 39- 055 42571 | Finland Oy Eli Lilly Finland Ab Tel: + 358-(0) 9 85 45 250 |
Cyprus Phadisco Ltd Tel: +357 22 715000 | Sweden Eli Lilly Sweden AB Tel: + 46-(0) 8 7378800 |
Latvia Eli Lilly (Suisse) S.A Parstavnieciba Latvija Tel: +371 67364000 |
Date of Last Revision of this Prospectus:
Other Sources of Information
Detailed information on this medicine is available on the European Medicines Agency website: http://www.ema.europa.eu, and on the website of the Spanish Agency for Medicines and Health Products (AEMPS) (http://www.aemps.gob.es/).
Instructions for Use Pre-filled multidose pen Each pen contains 4 fixed doses, one dose administered weekly. | |
| |
tirzepatide These instructions for use contain information on how to inject Mounjaro KwikPen
|
Important Information You Should Know Before Injecting Mounjaro KwikPen.
Read these instructions for use and the prospectus before starting to inject Mounjaro KwikPen and each time you get a new pen. There may be new information. This information does not replace the conversation with your doctor, nurse, or pharmacist about your medical condition or treatment.
Mounjaro KwikPen is a pre-filled multidose pen. The pen contains 4 fixed doses, one dose administered weekly.Inject one single injection weekly, under the skin (subcutaneously).
After 4 doses, discard (throw away) the pen, including any unused medicine. The pen will prevent you from dialing a full dose after 4 weekly doses have been administered. Do notinject the leftover medicine. Do not transfer the medicine from the pen to a syringe.
Do notshare your Mounjaro KwikPen with other people, even if the needle has been changed. You may give other people a serious infection or get a serious infection from them.
Blind or visually impaired people should not use the pen without the help of a trained person to use it.
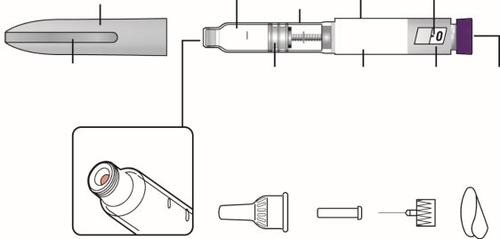
Parts Guide
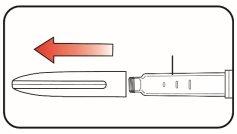
 Parts of Mounjaro KwikPen
Parts of Mounjaro KwikPen




Materials Needed for Injection
- Mounjaro KwikPen
- Needle compatible with KwikPen (If you do not know which needle to use for the pen, talk to your healthcare professional)
- Cotton swab, gauze, or cotton ball
- Container for disposing of sharp objects or household container
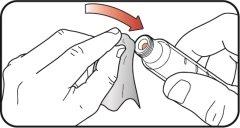
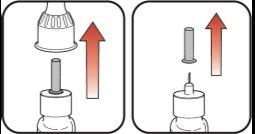
Preparation for Injecting Mounjaro KwikPen
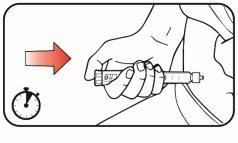
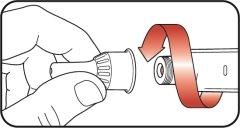
| Step 1:
| |
| Step 2:
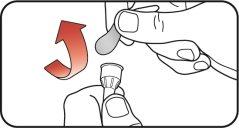
| |
| Step 3:
| |
| Step 4:
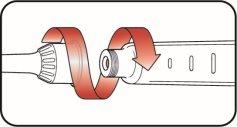
| |
| Step 5:
| |
| Step 6:
| |
|
| |
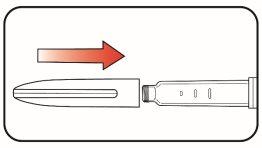
| Step 7:
| |
| Step 8:
| |
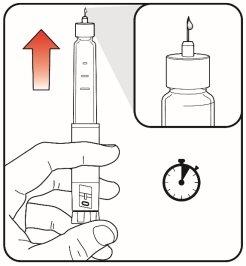
| Step 9:
The purging removes air from the cartridge and ensures the proper functioning of the pen. The pen has been purged if a small amount of medicine comes out of the pen's needle tip.
|
Injecting Mounjaro KwikPen
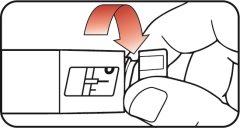
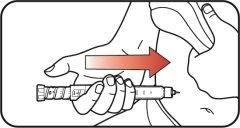
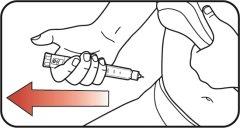
| Step 10:
|
| Step 11:
|
| Step 12:
|
|
|
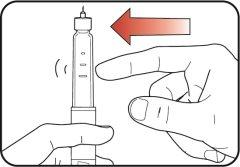
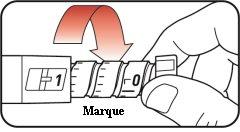
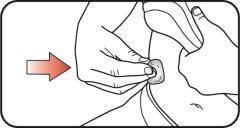
| Step 13:
If you see the icon in the window, it means you have received the full dose. If you do not see the icon in the dosing window, reinsert the needle into your skin and complete the injection. Do notredial the dose. If you still believe you have not received the full dose, do notstart again or repeat the injection. For more information, see the sections "Storing your Mounjaro KwikPen" or "Frequently Asked Questions". |
After Your Mounjaro KwikPen Injection
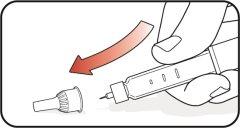
| Step 14:
| ||||||||||||||||||||||||||||||
| Step 15:
| ||||||||||||||||||||||||||||||
| Step 16:
Do notstore
Storing your Mounjaro KwikPen Unused pens: ? Store unused pensin the refrigeratorbetween 2°C and 8°C.
Used pens:
Disposal of Mounjaro KwikPen and pen needles
Frequently asked questions
Medical calendar
Last revised on |








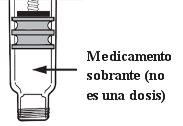 Remainingmedication:
Remainingmedication:





