
MONOPROST 50 micrograms/ml eye drops solution in single-dose containers


How to use MONOPROST 50 micrograms/ml eye drops solution in single-dose containers
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Monoprost 50 micrograms/ml eye drops, solution in single-dose container
Latanoprost
Read this package leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this package leaflet, you may need to read it again. If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the package leaflet
- What is Monoprost and what is it used for
- What you need to know before you use Monoprost
- How to use Monoprost
- Possible side effects
- Storage of Monoprost
- Contents of the pack and other information
1. What is Monoprost and what is it used for
Monoprost belongs to a group of medicines known as prostaglandins. It works by increasing the natural flow of fluid from inside the eye into the bloodstream.
Monoprost is used to treat conditions known as open-angle glaucoma and ocular hypertensionin adults. Both conditions are related to an increase in pressure inside the eye, which can affect vision.
2. What you need to know before you use Monoprost
Do not use Monoprost
- If you are allergic (hypersensitive) to latanoprost or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Talk to your doctor, pharmacist, or nurse before you start using Monoprost if you think any of the following apply to you:
- If you have had or are going to have eye surgery (including cataract surgery).
- If you have eye problems (such as eye pain, irritation, or inflammation, blurred vision).
- If you have severe asthma or uncontrolled asthma.
- If you use contact lenses. You can continue to use Monoprost, but you must follow the instructions in section 3 for contact lens users.
- If you have had or are having a viral infection of the eye caused by the herpes simplex virus (HSV).
Children
Monoprost has not been investigated in children (under 18 years).
Using Monoprost with other medicines
Monoprost may interact with other medicines. Tell your doctor or pharmacist what you are using, have recently used, or might use.
Pregnancy and breastfeeding
Do not use Monoprostif you are pregnant or breastfeeding.
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medicine.
Driving and using machines
When using Monoprost, blurred vision may occur for a short time. If this happens, do not driveor use tools or machines until your vision is clear again.
Important information about some of the ingredients of Monoprost
Monoprost containsmacrogolglycerol hydroxystearate (derived from castor oil), which may cause allergic reactions.
3. How to use Monoprost
Usual dose
- Follow your doctor's instructions for using Monoprost exactly. If you are unsure, consult your doctor or pharmacist again.
- The usual dose for adults (including the elderly) is one drop in the affected eye(s) once a day. It is best to administer it at night.
- Do not use Monoprost more than once a day, as the effectiveness of the treatment may decrease if it is administered more frequently.
- Follow the administration instructions for the medicine contained in this package leaflet or as indicated by your doctor. If you are unsure, ask your doctor, pharmacist, or nurse.
Contact lens users
If you use contact lenses, you must remove them before using Monoprost. After applying Monoprost, wait 15 minutes before putting your contact lenses back in.
Instructions for use
The eye drops are supplied in single-dose containers. The solution from one single-dose container of Monoprost should be used immediately after opening to treat the affected eye(s). Since sterility cannot be guaranteed after opening each single-dose container, a new container must be opened before each use, which must be discarded immediately after administration.
To use the eye drops, follow these instructions:
- Wash your hands and sit or stand comfortably.
- Open the pouch containing the single-dose containers. Note the date of first opening on the pouch.
- Separate one single-dose container from the strip.
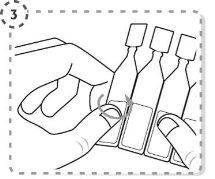
- Twist the tip of the single-dose container as shown. Do not touch the tip after opening the container.
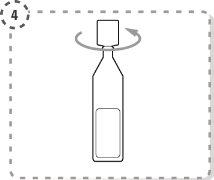
- Gently separate the lower eyelid of the affected eye with your finger.
- Place the tip of the single-dose container close to the eye, without touching it.
- Gently press the single-dose container so that only one drop falls into the eye, then remove your finger from the lower eyelid.
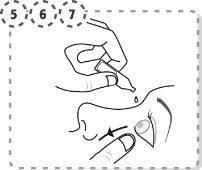
- Press the tip of the affected eye with your finger, near the nose. Apply pressure for 1 minute, keeping your eye closed.
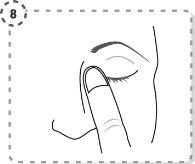
- Repeat the operation in the other eye, if your doctor has indicated it. Each single-dose container contains enough for both eyes.
- After use, discard the single-dose container. Do not save it for later use. Since sterility cannot be guaranteed after opening the single-dose container, a new container must be opened before each use.
If you use Monoprost with other eye drops
Wait at least 5 minutes between applying Monoprost and administering other eye drops.
If you use more Monoprost than you should
If you have applied more drops in the eye than you should, you may feel mild irritation in the eye, and your eyes may become red and tear; this situation should disappear, but if it concerns you, contact your doctor.
If you accidentally ingest Monoprost, consult your doctor as soon as possible.
If you forget to use Monoprost
Continue with the administration of the next dose as usual. Do not apply an extra drop in the eye to make up for the missed dose. If you are unsure, consult your doctor or pharmacist.
If you stop using Monoprost
If you want to stop using Monoprost, you must consult your doctor.
If you have any other questions about using this medicine, ask your doctor, pharmacist, or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
The following adverse reactions are known with the use of Monoprost:
Very common: may affect more than 1 in 10 patients.
- Gradual change in eye color due to an increase in the amount of brown pigment in the colored part of the eye, known as the iris.
- If you have mixed-color eyes (blue-brown, gray-brown, yellow-brown, or green-brown), you are more likely to notice this change than if your eyes are a single color (blue, gray, green, or brown).
- The change in eye color may take years to develop, although it can usually be seen after 8 months of treatment.
- The change in eye color may be permanent and may be more noticeable if Monoprost is used in only one eye.
- The change in eye color does not seem to be associated with the development of any problems.
- The change in eye color does not progress once treatment with Monoprost has been stopped.
- Redness of the eye.
- Ocular irritation (feeling of stinging, feeling of grit in the eye, itching, pain, and feeling of a foreign body in the eye).
- Gradual change in the eyelashes of the treated eye and the fine hair around the treated eye, observed in most patients of Japanese origin. These changes include an increase in color (darkening), length, thickness, and number of eyelashes.
Common: may affect up to 1 in 10 patients
- Irritation or erosion of the surface of the eye, inflammation of the eyelid (blepharitis), and eye pain, and sensitivity to light (photophobia), conjunctivitis.
Uncommon: may affect up to 1 in 100 patients
- Swelling of the eyelids, dry eye, inflammation or irritation of the surface of the eye (keratitis), blurred vision, inflammation of the colored part of the eye (uveitis), swelling of the retina (macular edema).
- Skin rash.
- Chest pain (angina), feeling the heartbeat (palpitations).
- Asthma, difficulty breathing (dyspnea).
- Chest pain.
- Headache, dizziness.
- Muscle pain, joint pain.
- Nausea, vomiting.
Rare: may affect up to 1 in 1,000 patients
- Inflammation of the iris (iritis), symptoms of swelling or injury/damage to the surface of the eye, swelling around the eye (periorbital edema), misdirected eyelashes or additional row of eyelashes, accumulation of fluid in the colored part of the eye (iris cyst).
- Reactions on the skin of the eyelids, darkening of the skin of the eyelids.
- Worsening of asthma.
- Intense itching of the skin.
- Development of a viral infection of the eye caused by the herpes simplex virus (HSV).
Very rare: may affect up to 1 in 10,000 patients
- Worsening of angina in patients who also have heart problems.
- Appearance of sinking of the eye (deepening of the eye sulcus).
Reporting of side effects
If you experience any side effects, talk to your doctor, pharmacist, or nurse, even if it is possible side effects not listed in this leaflet. You can also report side effects directly through the Spanish Pharmacovigilance System for Human Use Medicines: https//: notificaram.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Monoprost
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the packaging, on the outer packaging, and on the single-dose container. The expiry date is the last day of the month stated.
Store below 25°C.
After first opening the pouch: use the single-dose containers within the next 10 days.
After first opening the single-dose container: use immediately and discard the single-dose container after use.
Medicines should not be disposed of via wastewater or household waste. Place the containers and medicines you no longer need in the SIGRE collection point at the pharmacy. If you are unsure, ask your pharmacist how to dispose of the containers and medicines you no longer need. This will help protect the environment.
6. Contents of the pack and other information
Composition of Monoprost
The active substance is latanoprost.
1 ml of eye drops contains 50 micrograms of latanoprost.
The other ingredients (excipients) are: macrogolglycerol hydroxystearate 40, sorbitol, carbomer 974P, macrogol 4000, disodium edetate, sodium hydroxide (for pH adjustment), water for injections.
Appearance of the product and pack contents
This medicine is presented as eye drops, solution, in single-dose containers. The solution is slightly yellow and opalescent, without preservatives, contained in single-dose containers, presented in a pouch with 5 or 10 units. Each single-dose container contains 0.2 ml of eye drops solution.
The boxes contain 5 (1 x 5), 10 (2 x 5), 10 (1 x 10), 30 (6 x 5), 30 (3 x 10), 90 (18 x 5), or 90 (9 x 10) single-dose containers.
Not all pack sizes may be marketed.
Marketing authorization holder and manufacturer
Marketing authorization holder
LABORATOIRES THEA
12 RUE LOUIS BLERIOT
63017 CLERMONT-FERRAND CEDEX 2
FRANCE
Manufacturer
EXCELVISION
27, rue de la Lombardière
ZI la Lombardière
07100 ANNONAY
FRANCE
Or
LABORATOIRES THEA
12 RUE LOUIS BLERIOT
63017 CLERMONT-FERRAND CEDEX 2
FRANCE
Or
LABORATOIRE UNITHER
1 rue de l’Arquerie 50200 Coutances
FRANCE
Or
FAREVA Mirabel
Route de Marsat
Riom
63693 Clermont-Ferrand Cedex 9
FRANCE
Local representative
LABORATORIOS THEA, S.A.
C/ Enric Granados, nº 86-88, 2ª planta
08008 – Barcelona
This medicine is authorized in the Member States of the European Economic Area under the following names
Germany, Belgium, Bulgaria, Cyprus, Denmark, Spain, Finland, France, Greece, Netherlands, Iceland, Italy, Latvia, Luxembourg, Norway, Poland, Portugal, Sweden
Monoprost
Ireland Monopost Unidose
Austria, Slovenia, Lithuania, Czech Republic, Slovakia, United Kingdom, Romania
Monopost
Estonia…………………………………………………………………………………Monopro
Date of last revision of this package leaflet: May 2025
- Country of registration
- Average pharmacy price15.61 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to MONOPROST 50 micrograms/ml eye drops solution in single-dose containersDosage form: EYE DROP, 50 micrograms/mlActive substance: latanoprostManufacturer: Santen OyPrescription requiredDosage form: EYE DROP, 50 µg/mlActive substance: latanoprostManufacturer: Esteve Pharmaceuticals S.A.Prescription requiredDosage form: EYEDROP, 50 MG/MLActive substance: latanoprostManufacturer: Aurovitas Spain, S.A.U.Prescription required
Online doctors for MONOPROST 50 micrograms/ml eye drops solution in single-dose containers
Discuss questions about MONOPROST 50 micrograms/ml eye drops solution in single-dose containers, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















