MIRCERA 250 micrograms/0.3 ml SOLUTION FOR INJECTION IN PRE-FILLED SYRINGE


How to use MIRCERA 250 micrograms/0.3 ml SOLUTION FOR INJECTION IN PRE-FILLED SYRINGE
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Leaflet:information for the user
MIRCERA
30micrograms/0.3ml injectable solution in pre-filled syringe
50micrograms/0.3ml injectable solution in pre-filled syringe
75micrograms/0.3ml injectable solution in pre-filled syringe
100micrograms/0.3ml injectable solution in pre-filled syringe
120micrograms/0.3ml injectable solution in pre-filled syringe
150micrograms/0.3ml injectable solution in pre-filled syringe
200micrograms/0.3ml injectable solution in pre-filled syringe
250micrograms/0.3ml injectable solution in pre-filled syringe
360micrograms/0.6ml injectable solution in pre-filled syringe
methoxy polyethylene glycol-epoetin beta
Read the entire leaflet carefully before starting to use this medicine, as it contains important information for you.
- Keep this leaflet, as you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed to you only, and you should not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack:
- What MIRCERA is and what it is used for
- What you need to know before you start using MIRCERA
- How to use MIRCERA
- Possible side effects
- Storing MIRCERA
- Contents of the pack and further information
1. What MIRCERA is and what it is used for
Your doctor has prescribed this medicine because you are suffering from anaemia caused by chronic kidney disease. This anaemia is associated with typical symptoms such as fatigue, weakness, and shortness of breath. This means you have very few red blood cells and your haemoglobin level is too low (your body tissues may not be receiving enough oxygen).
MIRCERA is indicated only for the treatment of symptomatic anaemia associated with chronic kidney disease in adult and paediatric patients (from 3 months to less than 18 years of age) who are receiving maintenance treatment with an erythropoiesis-stimulating agent (ESA) after their haemoglobin levels have been stabilised with prior ESA treatment.
MIRCERA is a medicine produced by genetic engineering. Like the natural hormone erythropoietin, MIRCERA increases the number of red blood cells and the haemoglobin level in the blood.
2. What you need to know before you start using MIRCERA
Do not use MIRCERA
- if you are allergic to methoxy polyethylene glycol-epoetin beta or any of the other ingredients of this medicine (listed in section 6)
- if you have uncontrolled high blood pressure
Warnings and precautions
The safety and efficacy of MIRCERA treatment have not been established in other indications, including anaemia in patients with cancer.
The safety and efficacy of MIRCERA treatment in paediatric patients have only been established in patients whose haemoglobin level has been stabilised previously with an ESA.
Before starting treatment with MIRCERA
- In some patients treated with erythropoiesis-stimulating agents (ESAs), including MIRCERA, a disease called pure red cell aplasia (PRCA, a failure of the bone marrow to produce red blood cells) has been observed due to the presence of antibodies against erythropoietin.
- If your doctor suspects or confirms that you have these antibodies in your blood, you should not be treated with MIRCERA.
If you are a patient with hepatitis C and are receiving interferon and ribavirin, you should discuss this with your doctor, as the combination of ESAs with interferon and ribavirin can lead to a loss of effect and, in exceptional cases, the development of PRCA, a severe anaemia. ESAs are not approved for the treatment of anaemia associated with hepatitis C.
- If you are a patient with chronic kidney disease and anaemia, treated with an ESA, and are also a cancer patient, you should be aware that ESAs may have a negative impact on your disease. You should discuss other options for treating anaemia with your doctor.
- It is not known whether MIRCERA has a different effect in patients with haemoglobinopathies (disorders associated with abnormal haemoglobin levels), with bleeding, or with a high platelet count in the blood. If you have any of these conditions, your doctor will discuss this with you and treat you with caution.
- Healthy individuals should not use MIRCERA. Its use can lead to a haemoglobin level that is too high and cause heart or blood vessel problems that can be life-threatening.
During treatment with MIRCERA
- If you are a patient with chronic kidney disease, and in particular if you do not respond adequately to MIRCERA, your doctor will monitor your MIRCERA dose, as repeated increases in the MIRCERA dose if you are not responding to treatment may increase the risk of heart or blood vessel problems and may increase the risk of heart attack, stroke, and death.
- Your doctor may start treatment with MIRCERA if your haemoglobin level is 10 g/dl (6.21 mmol/l) or less. After starting treatment, your doctor will keep your haemoglobin level between 10 and 12 g/dl (7.45 mmol/l).
- Your doctor will check the amount of iron in your blood before and during treatment with MIRCERA. If the amount is too low, your doctor may give you additional treatment.
- Your doctor will check your blood pressure before and during treatment with MIRCERA. If your blood pressure is high and cannot be controlled, either by medication or by a special diet, your doctor will stop your treatment with MIRCERA or reduce the dose.
- Your doctor will check that your haemoglobin level does not exceed a certain value. A high haemoglobin level can increase the risk of serious heart or blood vessel problems, which can increase the risk of blood clots, including pulmonary embolism, heart attack, stroke, and death.
- Tell your doctor if you feel tired, weak, or short of breath, as this may mean that your treatment with MIRCERA is not effective. Your doctor will check that you do not have other causes of anaemia and may perform blood tests or examine your bone marrow. If you have developed PRCA, your treatment with MIRCERA will be stopped. You will not receive another ESA, and your doctor will treat this disease.
Children and adolescents
MIRCERA can be used to treat children and adolescents (from 3 months to less than 18 years of age) with anaemia associated with chronic kidney disease. They should be stabilised with maintenance ESA treatment before switching to MIRCERA and may or may not be receiving dialysis.
Ask your doctor, pharmacist, or nurse for advice before you or your child are given this medicine if you or your child are under 18 years of age.
Be careful with other medicines that stimulate the production of red blood cells:MIRCERA is one of the erythropoiesis-stimulating agents, like the human protein erythropoietin. Your doctor should always record the exact product you are using.
Severe skin reactions, such as Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN), have been observed with the administration of epoetins.
SJS/TEN may initially appear as red, circular patches on the skin, often with central blisters on the trunk. Ulcers on the mouth, throat, nose, genitals, and eyes (eye irritation and swelling) may also appear. These severe skin reactions are often preceded by fever or flu-like symptoms. The skin reaction may progress to widespread skin peeling and potentially life-threatening complications.
If you experience a severe skin reaction or any of these other skin symptoms, stop taking Mircera and contact your doctor or seek medical attention immediately.
Using MIRCERA with other medicines
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicines.
No interaction studies have been performed. There is no evidence that MIRCERA interacts with other medicines.
Using MIRCERA with food and drinks
Food and drinks do not affect MIRCERA.
Pregnancy, breastfeeding, and fertility
Ask your doctor or pharmacist for advice before taking any medicine.
No studies have been performed with MIRCERA in pregnant or breastfeeding women.
Tell your doctor if you are pregnant, think you may be pregnant, or plan to become pregnant. Your doctor will consider what is the best treatment for you during pregnancy.
Tell your doctor if you are breastfeeding or plan to breastfeed. Your doctor will advise you whether to stop breastfeeding or stop treatment with MIRCERA.
MIRCERA has not shown any effect on fertility in animal studies. The potential risk in humans is unknown.
Driving and using machines
MIRCERA does not affect your ability to drive or use machines.
Important information about some of the ingredients of MIRCERA
This medicine contains less than 1 mmol (23 mg) of sodium per ml; i.e., it is essentially “sodium-free”.
3. How to use MIRCERA
Follow the instructions for administration of this medicine exactly as told by your doctor. If you are unsure, ask your doctor or pharmacist again.
Your doctor will use the lowest effective dose to control the symptoms of your anaemia.
If you do not respond adequately to MIRCERA, your doctor will monitor your dose and inform you if you need to change the dose of MIRCERA.
Treatment with MIRCERA should be started under the supervision of a healthcare professional. The following injections can be administered by a healthcare professional or, once you have been taught, you (an adult patient) can inject MIRCERA yourself. Children and adolescents under 18 years of age should not inject MIRCERA themselves; administration should be performed by a healthcare professional or a trained adult caregiver. (Follow the instructions at the end of the leaflet on how to use the MIRCERA pre-filled syringe to inject yourself or another person).
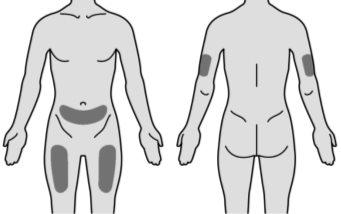
MIRCERA can be injected under the skin in the abdomen, arm, or thigh, or into a vein. Your doctor will decide what is best for you.
Your doctor will perform regular blood tests and monitor your haemoglobin level to see how your anaemia is responding to treatment.
- If you are an adult who is not currently being treated with an ESA
- If you are not on dialysis, the recommended initial dose of MIRCERA is 1.2 micrograms per kilogram of body weight, administered once a month in a single injection under the skin. Alternatively, your doctor may decide to administer an initial dose of MIRCERA of 0.6 micrograms per kilogram of body weight. The dose is administered once every two weeks under the skin or into a vein. Once your anaemia has been corrected, your doctor may modify the dosing regimen to once a month.
- If you are on dialysis, the recommended initial dose is 0.6 micrograms per kilogram of body weight. The dose is administered once every two weeks in a single injection, under the skin or into a vein. Once your anaemia has been corrected, your doctor may modify the dosing regimen to once a month.
Your doctor may increase or decrease your dose or temporarily stop your treatment to adjust your haemoglobin level to the appropriate level for you. Dose changes will not be made more frequently than once a month.
- If you are currently being treated with another ESA
Your doctor may replace your current medicine with MIRCERA. Your doctor will decide whether to treat you with MIRCERA administered in a single injection once a month. Your doctor will calculate your initial dose of MIRCERA based on your previous medicine. The first dose of MIRCERA will be administered on the day your previous medicine was scheduled.
Your doctor may increase or decrease your dose or temporarily stop your treatment to adjust your haemoglobin level to the appropriate level for you. Dose changes will not be made more frequently than once a month.
If you use more MIRCERA than you should
Tell your doctor or pharmacist if you use a higher dose of MIRCERA than you should, as you may need to have blood tests and your treatment may need to be stopped.
If you forget to use MIRCERA
If you forget a dose of MIRCERA, administer the dose as soon as you remember and ask your doctor when to administer the next doses.
If you stop treatment with MIRCERA
Treatment with MIRCERA is usually long-term. However, it may be stopped at any time if your doctor decides to do so.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
The frequency of possible side effects is listed below:
A common side effect (may affect up to 1 in 10 people) is hypertension (high blood pressure).
Uncommon side effects (may affect up to 1 in 100 people) are:
- headache
- thrombosis in the vascular access area (blood clots in the dialysis access) thrombocytopenia
- thrombosis
Rare side effects (may affect up to 1 in 1,000 people) are:
- hypertensive encephalopathy (very high blood pressure that can cause headache, especially sudden and severe migraine-like headache, confusion, speech disturbance, seizures, or convulsions)
- pulmonary embolism
- maculopapular rash (redness of the skin that can include pimples or pustules)
- flushing with warmth
- hypersensitivity (severe allergic reaction that can cause unusual sounds when breathing or difficulty breathing, swelling of the tongue, face, or throat, or swelling around the injection site, or make you feel dizzy, faint, or cause you to fall)
If you experience any of these symptoms, please tell your doctor immediately to receive treatment.
During clinical trials, patients experienced a small decrease in platelet count in the blood. Cases of low platelet count (thrombocytopenia) have been reported in the post-marketing period.
Hypersensitivity reactions, including cases of anaphylactic reaction and severe skin reactions such as Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN), have been observed with the administration of epoetins. These reactions can appear as red, circular patches on the skin, often with central blisters on the trunk, skin peeling, and ulcers on the mouth, throat, nose, genitals, and eyes, and may be preceded by fever and flu-like symptoms. Stop using Mircera if you experience these symptoms and contact your doctor or seek medical attention immediately. See also section 2.
As with other ESAs, cases of thrombosis, including pulmonary embolism, have been reported in the post-marketing period.
In some patients treated with ESAs, including MIRCERA, a disease called pure red cell aplasia (PRCA, a failure of the bone marrow to produce red blood cells) has been observed due to the presence of antibodies against erythropoietin.
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storing MIRCERA
Keep this medicine out of the sight and reach of children.
Do not use MIRCERA after the expiry date which is stated on the carton and on the label of the pre-filled syringe after “EXP”. The expiry date is the last day of the month shown.
Store in a refrigerator (2°C - 8°C). Do not freeze.
Keep the pre-filled syringe in the outer packaging to protect it from light.
You can take the MIRCERA pre-filled syringe out of the refrigerator and store it at room temperature (not above 30°C) for a single period of one month. During this period, you must not put MIRCERA back in the refrigerator before using it. Once you have taken the medicine out of the refrigerator, you must use it within this one-month period.
Only inject solutions that are clear, colourless to slightly yellowish, and free from visible particles.
Medicines should not be disposed of via wastewater or household waste.
Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Container Contents and Additional Information
Composition of MIRCERA
- The active ingredient is methoxy polyethylene glycol epoetin beta. A pre-filled syringe contains: 30, 50, 75, 100, 120, 150, 200 or 250 micrograms in 0.3 ml and 360 micrograms in 0.6 ml.
- The other components are monosodium phosphate monohydrate, sodium sulfate, mannitol (E421), methionine, poloxamer 188, and water for injectable preparations.
Appearance of the Product and Container Contents
MIRCERA is an injectable solution in a pre-filled syringe.
Clear solution from colorless to slightly yellowish and without visible particles.
MIRCERA is presented in pre-filled syringes with a laminated piston and a protector with a 27 G1/2 needle. Each pre-filled syringe contains 0.3 ml or 0.6 ml of solution. The pre-filled syringes are not designed for partial dose administration. MIRCERA is available, for all doses, in packs of 1 and also in packs of 3 for the 30, 50, 75 microgram/0.3 ml doses. Only some pack sizes may be marketed.
Marketing Authorization Holder and Manufacturer
Roche Registration GmbH
Emil-Barell-Strasse 1
79639 Grenzach-Wyhlen
Germany
Manufacturer
Roche Pharma AG
Emil-Barell-Strasse 1
79639 Grenzach-Wyhlen
Germany
You can request more information about this medicinal product by contacting the local representative of the marketing authorization holder:
|
Czech Republic Roche s.r.o. Tel: +420 - 2 20382111 | Hungary Roche (Hungary) Kft. Tel: +36 - 1 279 4500 |
Denmark Roche Pharmaceuticals A/S Tel: +45 - 36 39 99 99 | Malta (See Ireland) |
Germany Roche Pharma AG Tel: +49 (0) 7624 140 | Netherlands Roche Nederland B.V. Tel: +31 (0) 348 438050 |
Estonia Roche Eesti OÜ Tel: + 372 - 6 177 380 | Norway Roche Norge AS Tel: +47 - 22 78 90 00 |
Greece Roche (Hellas) A.E. Tel: +30 210 61 66 100 | Austria Roche Austria GmbH Tel: +43 (0) 1 27739 |
Spain Roche Farma S.A. Tel: +34 - 91 324 81 00 | Poland Roche Polska Sp.z o.o. Tel: +48 - 22 345 18 88 |
France Roche Tel: +33 (0)1 47 61 40 00 | Portugal Roche Farmacêutica Química, Lda Tel: +351 - 21 425 70 00 |
Croatia Roche d.o.o. Tel: + 385 1 47 22 333 | Romania Roche România S.R.L. Tel: +40 21 206 47 01 |
Ireland Roche Products (Ireland) Ltd. Tel: +353 (0) 1 469 0700 | Slovenia Roche farmacevtska družba d.o.o. Tel: +386 - 1 360 26 00 |
Iceland Roche Pharmaceuticals A/S c/o Icepharma hf Tel: +354 540 8000 | Slovak Republic Roche Slovensko, s.r.o. Tel: +421 - 2 52638201 |
Italy Roche S.p.A. Tel: +39 - 039 2471 | Finland Roche Oy Tel: +358 (0) 10 554 500 |
Cyprus Γ.Α.Σταμ?της & Σια Λτδ. Tel: +357 - 22 76 62 76 | Sweden Roche AB Tel: +46 (0) 8 726 1200 |
Latvia Roche Latvija SIA Tel: +371 - 6 7039831 | United Kingdom(Northern Ireland) Roche Products (Ireland) Ltd. Tel: +44 (0) 1707 366000 |
Date of Last Revision of this Leaflet:
Detailed information on this medicinal product is available on the European Medicines Agency website http://www.ema.europa.eu/.
MIRCERA Pre-filled Syringe Instructions for Use |
The following instructions explain how to use MIRCERA pre-filled syringes so that you or another person can administer an injection. It is important that you read and follow these instructions carefully so that you can use the pre-filled syringe correctly and safely. Do notattempt to administer an injection until you are sure you understand how to use the pre-filled syringe. If in doubt, consult a healthcare professional. Children and adolescents under 18 years of age must notself-inject MIRCERA; administration should be performed by a healthcare professional or a trained adult caregiver. Always follow the instructions in these Instructions for Use, as they may differ from your experience. These instructions will help you prevent incorrect treatment or risks such as needlestick injuries or early activation of the needle safety device, or problems related to needle placement. |
IMPORTANT INFORMATION
? Use MIRCERA pre-filled syringe only if you have been prescribed this medication.
? Read the packaging and make sure you have the dose prescribed by your doctor.
? Do not useMIRCERA if the syringe, needle, box, or plastic tray containing the syringe appears to be damaged.
- The needle is fragile; handle it with care.
? Do not touchthe activation protectors (see Figure A) as this may damage the syringe and render it unusable.
? Do not usethe syringe if the contents are cloudy, whitish, or contain particles.
? Never attempt to disassemble the syringe.
? Never throw or handle the syringe by the plunger.
? Do not removethe needle protector until you are ready to administer the injection.
? Do not ingestthe medication from the syringe.
? Do not injectthrough clothing.
? Do notreuse or re-sterilize the syringe or needle.
? Pre-filled syringes are not designed to administer partial doses.
? Keep the syringe, needle, and supplies out of the reach of children.
STORAGE
Keep the pre-filled syringe, needle, andcontainer for disposing of sharp objectsout of the reach of children.
Store the syringe and needle in their original packaging until the time of use.
Always store the syringe and needle in the refrigerator at a temperature of 2 – 8 ºC (35.6 - 46.4°F).
Do notallow the medication to freeze, and protect the medication and needle from light. Keep the syringe and needle in a dry place.
MATERIALS INCLUDED IN THE PACKAGING (Figure A):
- A pre-filled syringe containing MIRCERA
- A needle for injection
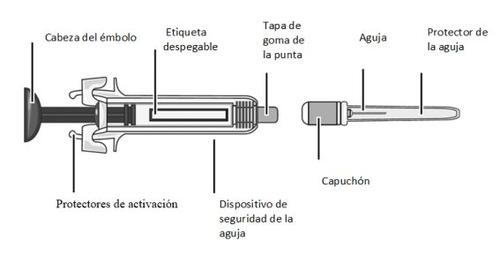
Figure A
MATERIALS NOT INCLUDED IN THE PACKAGING (Figure B):
Alcohol wipes | Sterile cotton or gauze | Containers for disposing of sharp objects for the safe disposal of used needles and syringes |
Figure B
Place all the items you need for an injection on a flat, clean, and well-lit surface, such as a table.
HOW TO ADMINISTER THE INJECTION | |
Step 1: Allow the syringe to reach room temperature | |
Figure C | Carefully remove the MIRCERA pre-filled syringe packaging from the refrigerator. Keep the syringe and needle inside the packaging, protected from light, and let it reach room temperature for at least 30 minutes (Figure C). ? If the medication is not allowed to reach room temperature, the injection may be uncomfortable and may make it difficult to push the plunger. ? Do notheat the syringe in any other way. |
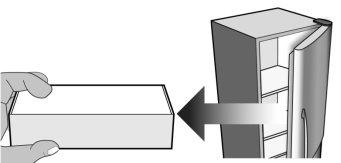
Figure D | Open the box and remove the plastic tray of MIRCERA pre-filled syringe from the packaging without removing the protective film (Figure D). |
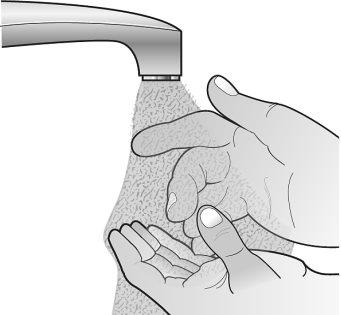
Step 2: Wash your hands | ||
| ||
Step 3: Remove the pre-filled syringe and inspect it visually | ||
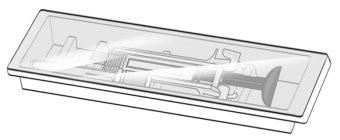
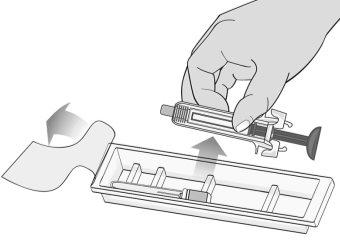
Figure F | Remove the protective film from the plastic tray and remove the packaged needle and syringe by holding the syringe by the middle of the body without touching the activation protectors (Figure F). ? Only handle the syringe by the body, as contact with the activation protectors can cause premature release of the safety device. |
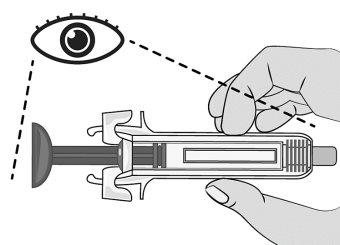
Figure G | Inspect the syringe for damage and check the expiration date indicated on the syringe and packaging. This is important to ensure that the syringe and medication are safe for use (Figure G). Do notuse the syringe if: ? It has accidentally fallen. ? Any part of the syringe appears to be damaged. ? The contents are cloudy, whitish, or contain particles.
? The expiration date has passed. |
Step 4: Attach the needle to the syringe
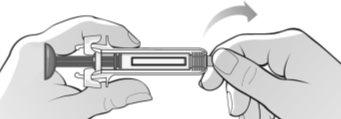
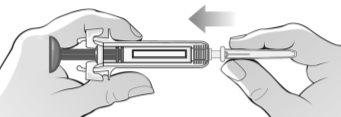
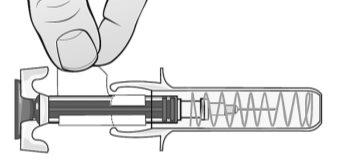
Figure H | Hold the syringe firmly by the middle and the rubber cap of the tip and remove the cap from the syringe (bend and pull) (Figure H).
? Do not touchthe activation protectors. ? Do not pushthe plunger. ? Do not pullthe plunger. | |||
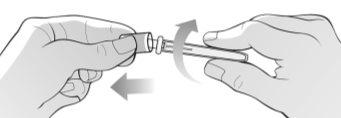
Figure I | Hold the packaged needle firmly with both hands and check if the packaged needle is damaged. Break the seal of the needle using a twisting motion and remove the needle cap as indicated in the illustration (Figure I). Immediately discard the needle cap in the container for disposing of sharp objects. Do not removethe needle protector that performs this function. Do not use the needle if:
| |||
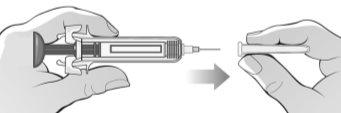
Figure J | Attach the needle to the syringe, pushing it firmly until it clicks and twisting or turning it slightly (Figure J). | |||
Step 5: Remove the syringe protector and prepare for injection | ||||
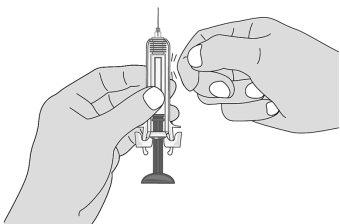
Figure K | Hold the syringe firmly with one hand by the middle of the body and pull the syringe protector with the other hand. Discard the syringe protector in the container for disposing of sharp objects (Figure K).
| |||
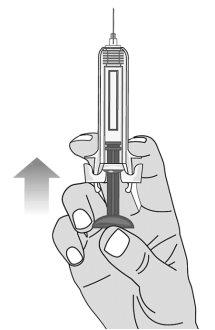
Figure L | To remove air bubbles from the pre-filled syringe, hold the syringe with the needle facing upwards. Gently tap the syringe to make the air bubbles rise (Figures L and M). | |||
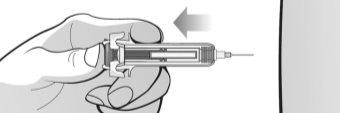
Figure M | Slowly push the plunger to remove all the air, as instructed by the healthcare professional (Figure M). | |||
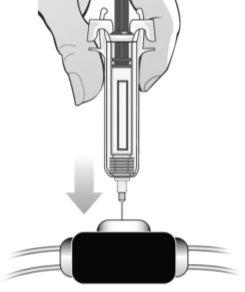
Step 6: Administer the injection There are two different ways (routes) to inject MIRCERA into your body. Follow the recommendations given by the healthcare professional on how to inject MIRCERA. | ||||
Subcutaneous route: If you have been advised to inject MIRCERA under the skin, administer the dose as described below. | ||||
Figure N | Choose one of the recommended injection sites indicated. You can inject MIRCERA into the upper arm, thigh, or abdomen, except for the area around the navel (Figure N). The back of the upper arm is not a recommended injection site for self-injection. Use this injection site only if you are administering the injection to another person. When selecting an injection site: ? Choose a different injection site each time you administer an injection, at a distance of at least three centimeters from the area where you administered the previous injection. ? Do notinject into areas that may be irritated by a belt or the waistband of your clothing. ? Do notinject into moles, scars, bruises, or areas where the skin is sensitive, red, hard, or damaged. | |||
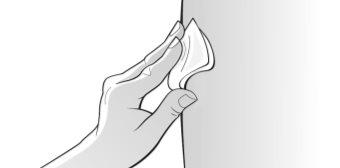
Figure O | Clean the chosen injection site with an alcohol wipe to reduce the risk of infection; follow the instructions on the alcohol wipe carefully (Figure O). ? Let the skin dry for about 10 seconds. ? Make sure notto touch the cleaned area before injection and do not blow on it. ? Immediately discard the alcohol wipe. | |||
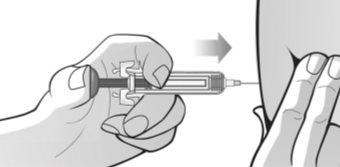
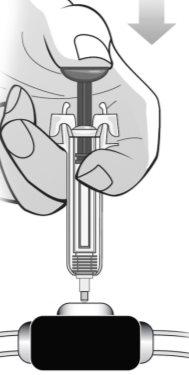
Figure P | Assume a comfortable position before administering the MIRCERA injection. To ensure that the needle can be inserted correctly into the skin, pinch a fold of skin at the injection site with your free hand. It is essential to pinch your skin to ensure that you inject under the skin (into fatty tissue) but not deeper (into the muscle). If the injection is administered into the muscle, it may be uncomfortable (Figure P). Carefully insert the needle completely into the skin at a 90° angle, making a quick motion as if throwing a dart. Then, hold the syringe in place and stop pinching the skin. Do notmove the needle while it is inserted into the skin. Once the needle is completely inserted into the skin, slowly push the plunger with your thumb and against the grips until all the medication is injected while holding the syringe with your index and middle fingers. The plunger rod should be completely down (pressed) and you should hear a "click" that indicates the activation of the needle safety device (Figure Q). | |||
| Do notrelease the plunger before completing the injection or before pressing the plunger completely. Remove the syringe from the skin, WITHOUTreleasing the plunger (Figure R). | |||
Figure R | Release the plunger, allowing the needle safety device of the syringe to protect the needle (Figure S). | |||
Figure T | You can now remove the peel-off label if necessary (Figure T). | |||
After injection: ? Place a sterile cotton ball or gauze over the injection site and press for several seconds. ? Discard the cotton ball or gauze immediately after use. ? Do notrub the injection site with a dirty hand or cloth. ? If necessary, you can cover the injection site with a small bandage. Discard the syringe: ? Do notattempt to replace the needle protector. ? Do notreuse or re-sterilize the syringe and/or needle. ? Do notthrow away the used syringe with the needle in the household trash. ? Throw away used syringes in a sharps disposal container and/or according to the regulations of the health authorities. ? Discard the full sharps disposal container. INTRAVENOUS ROUTE: If the healthcare professional has recommended that you inject MIRCERA into a vein, you should follow the process described below. After preparing the syringe according to the description of steps 1 to 5: Clean the venous port of the dialysis tube with an alcohol swab as indicated by the supplier or manufacturer. Discard the alcohol swab immediately after use.
Step 7: Discard the used syringe with the needle | ||||
| ||||
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to MIRCERA 250 micrograms/0.3 ml SOLUTION FOR INJECTION IN PRE-FILLED SYRINGEDosage form: INJECTABLE, 100 µgActive substance: methoxy polyethylene glycol-epoetin betaManufacturer: Roche Registration GmbhPrescription requiredDosage form: INJECTABLE, 120 µgActive substance: methoxy polyethylene glycol-epoetin betaManufacturer: Roche Registration GmbhPrescription requiredDosage form: INJECTABLE, 150 µgActive substance: methoxy polyethylene glycol-epoetin betaManufacturer: Roche Registration GmbhPrescription required
Online doctors for MIRCERA 250 micrograms/0.3 ml SOLUTION FOR INJECTION IN PRE-FILLED SYRINGE
Discuss questions about MIRCERA 250 micrograms/0.3 ml SOLUTION FOR INJECTION IN PRE-FILLED SYRINGE, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions