
MINIMS TROPICAMIDE 10 mg/ml EYE DROPS SOLUTION IN SINGLE-DOSE CONTAINERS

How to use MINIMS TROPICAMIDE 10 mg/ml EYE DROPS SOLUTION IN SINGLE-DOSE CONTAINERS
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Minims Tropicamide 10 mg/ml Eye Drops Solution in Single-Dose Container
tropicamide
Read this entire leaflet carefully before starting to use this medicine, as it contains important information for you.
- Keep this leaflet, as you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed to you only, and you should not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you experience any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the Package Leaflet
- What Minims Tropicamide is and what it is used for
- What you need to know before you start using Minims Tropicamide
- How to use Minims Tropicamide
- Possible side effects
- Storage of Minims Tropicamide
- Contents of the pack and further information
1. What Minims Tropicamide is and what it is used for
Minims Tropicamide eye drops are a medicine that is applied topically to the eyes. This medicine contains the active ingredient tropicamide. It is used to dilate the pupil of the eye and temporarily paralyze the internal muscles of the eye. Your doctor or ophthalmologist may use it to examine your eye or to facilitate ocular surgery procedures.
Minims Tropicamide can be used alone or in combination with other ophthalmic medications.
Minims Tropicamide is indicated for use in adults (including the elderly), adolescents, and children over 6 years of age.
2. What you need to know before you start using Minims Tropicamide
Do not use Minims Tropicamide:
- If you are allergic to tropicamide or any of the other ingredients of this medicine (listed in section 6);
- If you have or think you may have glaucoma. This is especially important in elderly patients.
Do not use this medicine if any of the above applies to you. If you are not sure, talk to your doctor or pharmacist before using Minims Tropicamide.
Warnings and precautions
Talk to your doctor or pharmacist before using Minims Tropicamide:
- If you have red eyes (inflammation symptoms);
- If you notice signs of skin irritation or allergic skin reaction after using this medicine;
- If you have increased pressure inside the eye;
- If you have kidney or liver failure;
- If you cannot use medications called tropane alkaloids (belladonna alkaloids), as you may be more likely to experience side effects.
Be careful if Minims Tropicamide is administered to children. If this medicine is used incorrectly for a long time, it can be absorbed into the systemic circulation, especially in children. Close the eyelids and press gently on the inner corner of the eyelid for 2 minutes to reduce systemic absorption. This will prevent the solution from going into the nose and throat. This is especially recommended in children.
Children and adolescents
Talk to your doctor about using Minims Tropicamide in children and adolescents, so they can decide if this medicine is suitable for you or your child.
Minims Tropicamide is not recommended for use in children under 6 years of age.
Other medicines and Minims Tropicamide
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicines.
This is especially important if you are taking medicines for the treatment of depression (antidepressants), anxiety (central nervous system depressants), and heart diseases (hypotensives and cardiovascular medications).
No interactions have been reported between tropicamide used at the ocular level and other medicines. However,
Minims Tropicamide may enhance the effect of other medicines of the same class (mydriatics and cycloplegics).
If you use Minims Tropicamide with other topical ophthalmic products, you should wait at least 5 minutes between the administration of each medicine. Ophthalmic gels and ointments should always be administered last.
Pregnancy, breastfeeding, and fertility
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctor or pharmacist for advice before using this medicine.
Your doctor may decide to prescribe this eye drop or prescribe an alternative.
It is not known whether tropicamide is excreted in human breast milk after ocular use.
There are no data on the effects of tropicamide on fertility in humans.
Driving and using machines
This medicine may cause blurred vision and sensitivity to light. This can affect your depth perception and, therefore, has a negative influence on your ability to drive. Do not drive or use machines until your vision is clear.
3. How to use Minims Tropicamide
Follow the instructions for administration of this medicine exactly as indicated by your doctor or pharmacist. If you are unsure, talk to your doctor or pharmacist again.
This medicine should not be used for longer than recommended by your doctor. This medicine can be used alone or in combination with other ophthalmic medications.
This medicine should be used locally in the eyes. You can use a mirror to help you see what you are doing.
The normal dose for adults (including the elderly), adolescents, and children from 6 years of age is 1 drop, followed by a second drop after a 5-minute interval. It may be necessary to instill 1 more drop after 30 minutes.
Instructions for use:
- Wash your hands.
- Remove contact lenses (if applicable).
- Gently dry the eye with a paper towel. Unscrew and pull off the cap to separate it from the Minims Tropicamide body (Figure 1).
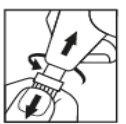
Figure 1
- Gently pull down your lower eyelid and carefully apply 1 drop inside the lower eyelid (Figures 2 and 3). Avoid touching the eye with the tip of the container to avoid damaging the eye and contaminating the solution.


Figure 2 Figure 3
- Close your eyelids and press gently on the inner corner of the eyelid for 2 minutes. This will prevent the solution from going into the nose and throat (Figure 4).
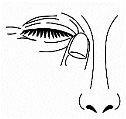 Figure 4
Figure 4
- Repeat steps 4 and 5 in the other eye if necessary; then discard the unit, even if some solution remains.
- Wash your hands after using the product.
The eye drops are for single use and are provided in single-use units, for use in both eyes if necessary. Once the single-dose container is opened, it should be used immediately. Discard any unused portion.
If you use Minims Tropicamide with other topical ophthalmic products, you should wait at least 5 minutes between the administration of each medicine. Ophthalmic gels and ointments should always be administered last.
Use in children under 6 years of age
Minims Tropicamide is not recommended for use in children under 6 years of age.
If you use more Minims Tropicamide than you should
Overdose is unlikely to occur.
Symptoms of overdose may include: redness and dryness of the skin (rash may occur in children), blurred vision, rapid and irregular pulse, fever, abdominal swelling in infants, convulsions, hallucinations, or loss of coordination.
In case of overdose or accidental ingestion, talk to your doctor or pharmacist immediately or call the Toxicology Information Service, phone: 91 562 04 20, indicating the medicine and the amount ingested. Go to the hospital, as serious adverse reactions can occur (especially in children).
If you forget to use Minims Tropicamide
Do not apply a double dose to make up for the forgotten dose.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Very common:may affect more than 1 in 10 people
- Eye pain
- Blurred vision
Frequency not known:cannot be estimated from the available data
- Photophobia (sensitivity to light)
- Ocular itching (transient)
- Increased pressure inside the eye
- Dry mouth
- Allergic reactions (hypersensitivity)
- Nausea
- Dizziness
- Headache
- Fainting
- Decrease in blood pressure
Among the systemic side effects of tropicamide, the following can be included: skin redness, dryness of the skin, alterations in heart rate, decreased sweating, decreased gastrointestinal motility and constipation, inability to empty the bladder completely, and decreased nasal, bronchial, and tear secretions.
Additional side effects in children
Very common:may affect more than 1 in 10 people
- Blurred vision
Frequency not known:cannot be estimated from the available data
- Increased pressure inside the eye
With this class of drugs, changes in behavior, confusion, excitement, hallucinations, increased somnolence, rapid breathing, and changes in pulse rate have been reported, especially in children, which require counseling and urgent medical attention.
Reporting of side effects
If you experience any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly through the Spanish Medicines Agency's website: https://www.notificaram.es/. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Minims Tropicamide
Keep this medicine out of the sight and reach of children.
Do not store above 25°C. Do not freeze.
Store in the original packaging to protect from light and moisture.
Do not use this medicine after the expiry date which is stated on the carton and on the sachet after EXP. The expiry date is the last day of the month shown.
Do not use this medicine if you notice any visible signs of deterioration.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help protect the environment.
6. Contents of the pack and further information
Minims Tropicamide content
- The active ingredient is tropicamide. One ml of the eye drops solution contains 10 mg of tropicamide.
- The other ingredients are sodium hydroxide, hydrochloric acid, and purified water.
Appearance of the product and pack contents
Minims Tropicamide is a clear, colorless, sterile, and single-use solution, which is practically free from visible particles and is presented in a conical-shaped container with a screw cap. Each unit is wrapped in a polypropylene/paper pouch. Each container holds approximately 0.5 ml of solution.
Each carton contains 20 single-dose sterile containers of 0.5 ml.
Marketing authorization holder and manufacturer
Marketing authorization holder
BAUSCH + LOMB IRELAND LIMITED
3013 Lake Drive
Citywest Business Campus
Dublin 24, D24PPT3
Ireland
Manufacturer
Laboratoire Chauvin
Zone Industrielle de Ripotier, 50 Avenue Jean Monnet,
07200, Aubenas, France
You can obtain further information on this medicine by contacting the local representative of the marketing authorization holder.
Local representative in Spain
Bausch & Lomb S.A.
Avda. Valdelaparra, nº 4, 28108 Alcobendas
Madrid, Spain.
Tel: 91 – 657 63 00
This medicine is authorized in the Member States of the European Economic Area under the following names:
Hungary: Minims Tropicamide 10 mg/ml oldatos szemcsepp egyadagos tartályban Greece: Tropicamide/Bausch+Lomb Cyprus: Tropicamide/Bausch+Lomb Portugal: Minims Tropicamida 10 mg/mL, colírio, solução em recipiente unidose Spain: Minims Tropicamida 10 mg/ml colirio en solución en envase unidosis |
This leaflet was last revised in December 2021
Detailed information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) https://www.aemps.gob.es/
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to MINIMS TROPICAMIDE 10 mg/ml EYE DROPS SOLUTION IN SINGLE-DOSE CONTAINERSDosage form: EYEDROP, 10 mg/mlActive substance: tropicamideManufacturer: Alcon Healthcare S.A.Prescription requiredDosage form: EYEDROP, -Active substance: atropineManufacturer: Alcon Healthcare S.A.Prescription requiredDosage form: EYEDROP, 5 mgActive substance: atropineManufacturer: Alcon Healthcare S.A.Prescription required
Online doctors for MINIMS TROPICAMIDE 10 mg/ml EYE DROPS SOLUTION IN SINGLE-DOSE CONTAINERS
Discuss questions about MINIMS TROPICAMIDE 10 mg/ml EYE DROPS SOLUTION IN SINGLE-DOSE CONTAINERS, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















