
MINIMS PREDNISOLONE 5 MG/ML EYE DROPS IN SOLUTION IN SINGLE-DOSE CONTAINERS

How to use MINIMS PREDNISOLONE 5 MG/ML EYE DROPS IN SOLUTION IN SINGLE-DOSE CONTAINERS
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Leaflet: Information for the user
Minims Prednisolone 5 mg/ml eye drops in single-dose container
sodium prednisolone phosphate
Read this leaflet carefully before starting to use this medicine, as it contains important information for you.
- Keep this leaflet, as you may need to read it again.
- If you have any doubts, consult your doctor or pharmacist.
- This medicine has been prescribed to you only, and you should not give it to others, even if they have the same symptoms, as it may harm them.
- If you experience any type of adverse effect, consult your doctor or pharmacist, even if it is a possible adverse effect that is not listed in this leaflet. See section 4.
Contents of the leaflet
- What Minims Prednisolone is and what it is used for
- What you need to know before using Minims Prednisolone
- How to use Minims Prednisolone
- Possible side effects
- Storage of Minims Prednisolone
- Contents of the pack and further information
1. What Minims Prednisolone is and what it is used for
Minims Prednisolone contains sodium prednisolone phosphate, which belongs to a group of medicines called corticosteroids. It is used to treat eye inflammation (redness and pain in the eyes) when it is not caused by an infection. The affected part of the eye varies depending on the cause of the inflammation.
This medicine is indicated for adults.
Minims Prednisolone is not suitable for the treatment of eye infections.
2. What you need to know before using Minims Prednisolone
Do not use Minims Prednisolone:
- if you are allergic to prednisolone or any of the other components of this medicine (listed in section 6).
- if you have an eye infection caused by a virus, fungus, or bacteria.
Warnings and precautions
Consult your doctor or pharmacist if you notice blurred vision or visual disturbances.
You should not wear contact lenses when using this medicine, as its use may increase the risk of infection.
Prolonged use may cause an increase in pressure inside the eye. You should not use this medicine if you are at risk of developing glaucoma.
Children and adolescents
This medicine should not be used in children and adolescents.
Other medicines and Minims Prednisolone
Tell your doctor or pharmacist if you are using, have recently used, or may need to use any other medicine, including those obtained without a prescription.
Minims Prednisolone may increase the effects of:
- medicines used to treat epilepsy (barbiturates)
- medicines used to help you sleep or relieve anxiety (sedative hypnotics)
- medicines used to treat depression (tricyclic antidepressants)
Minims Prednisolone may decrease the effects of:
- medicines such as anticholinesterases (used to reduce intestinal movement and for myasthenia gravis, a disease that weakens the muscles).
- medicines used to treat viral eye infections.
- medicines similar to aspirin, called salicylates (used for inflammation, pain, fever, and to prevent excessive blood clotting).
Other medicines may increase the effects of Minims Prednisolone, and your doctor may want to monitor you closely if you are taking them (including some HIV medicines, such as cobicistat).
If used in combination with other ophthalmic preparations, an interval of at least 5 minutes should be left between successive applications. Ophthalmic gels and ointments should always be administered last.
Pregnancy, breastfeeding, and fertility
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor before using this medicine.
Driving and using machines
You may notice blurred vision for a short period immediately after applying Minims prednisolone. If this occurs, wait until your vision is clear before driving or using machinery.
Excipients
This medicine contains 0.07 mg of phosphates in each drop, which is equivalent to 0.91 mg/0.5 ml.
If you have severe damage to the transparent layer of the front of the eye (cornea), phosphates may cause (in very rare cases) opaque spots on the cornea due to calcium deposits during treatment.
3. How to use Minims Prednisolone
Follow the instructions for using this medicine exactly as stated in this leaflet or as directed by your doctor or pharmacist. If you are unsure, ask your doctor or pharmacist.
Remember:Minims Prednisoloneshould only be applied to the eyes. The drops are for single use and are provided in single-dose units containing 13.5 drops, which are sufficient to treat both eyes if necessary. Once opened, use immediately. Discard any unused portion.
The recommended dose is:
One drop in one or both eyes with the frequency indicated by your doctor. It is usually administered one drop 4 times a day for 1 to 3 weeks and then one drop twice a day. In more severe cases, your doctor may indicate that you start with one drop 8 times a day during the first week.
Method of administration (application of Minims Prednisoloneeye drops)
If you are going to put the eye drops in yourself, follow these instructions carefully. To facilitate the correct application of the eye drops, it may be helpful to sit in front of a mirror so that you can see what you are doing.
- Wash your hands.
- Gently clean the area around your eyes with a cloth to remove any moisture.
- Open the single-dose container (image 1).
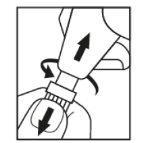
Image 1
- Gently pull the lower eyelid down (image 2).

Image 2
- Carefully place one drop inside the middle area of the lower eyelid. Be careful not to touch your eye with the container (image 3).

Image 3
- Release the lower eyelid and gently press the inner corner of the eye against the bridge of the nose for 1 or 2 minutes. While your finger presses the nose, blink slowly a few times to spread the drop across the entire surface of the eye (image 4).
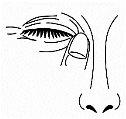
Image 4
- Wipe away any excess medicine with a cloth.
- Repeat these steps in the other eye if necessary; then discard the container, even if some solution remains.
If you use more Minims Prednisolone than you should:
If you administer too much Minims Prednisolone to your eye, you are unlikely to have any problems. If you have any doubts, consult your doctor. If you accidentally ingest Minims Prednisolone or feel unwell after using this medicine, contact your doctor or go to the nearest hospital emergency department immediately.
In case of overdose or accidental ingestion, contact your doctor or call the Toxicology Information Service, phone 91 562 04 20, indicating the medicine and the amount ingested.
If you forget to use Minims Prednisolone
Use the eye drops as soon as you remember and apply the next dose at the usual time. Do not administer a double dose to make up for the missed dose.
If you stop using Minims Prednisolone
If you have any further questions about using this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everyone gets them.
Very common side effects (may affect up to 1 in 10 people):
Increased eye pressure.
Very rare side effects (may affect up to 1 in 10,000 people):
Corneal calcification has been reported in patients with significantly damaged corneas.
Side effects of unknown frequency
Blurred vision.
Prolonged and frequent use of corticosteroids may cause clouding of the lens (cataract formation).
Secondary glaucoma, a disease that can damage the optic nerve and cause vision loss, may occur as a result of increased eye pressure.
Consult your doctor if you notice any change in your vision.
Reporting side effects
If you experience any type of side effect, consult your doctor or pharmacist, even if it is a possible side effect that is not listed in this leaflet. You can also report them directly through the Spanish Pharmacovigilance System for Human Use Medicines: https://www.notificaram.es/.
By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Minims Prednisolone
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiration date stated on the carton and on the pouch of the container, after "EXP".
Use the single-dose container once and discard any remaining solution.
Store below 25°C. Do not refrigerate or freeze. Keep in the original packaging and keep the blister pack in the outer packaging, as the product is very sensitive to light.
Medicines should not be disposed of via wastewater or household waste. Place the containers and medicines you no longer need in the SIGRE collection point at the pharmacy. If you have any doubts, ask your pharmacist how to dispose of the containers and medicines you no longer need. This will help protect the environment.
6. Contents of the pack and further information
Composition of Minims Prednisolone
- The active ingredient is sodium prednisolone phosphate
- The other ingredients are: disodium edetate, sodium dihydrogen phosphate dihydrate, sodium chloride, sodium hydroxide (to adjust the pH), purified water
Appearance of the product and contents of the container
Minims Prednisolone is presented in a 0.5 ml polypropylene container, conical in shape, with a screw cap. Each single-dose container is wrapped in an individual polypropylene/paper pouch.
Each box contains 20 single-dose containers.
Marketing authorization holder and manufacturer
Marketing authorization holder
BAUSCH + LOMB IRELAND LIMITED
3013 Lake Drive
Citywest Business Campus
Dublin 24, D24PPT3
Ireland
Manufacturer
Laboratoire Chauvin
Zone Industrielle de Ripotier
50 Avenue Jean Monnet
07200 Aubenas
France
You can request more information about this medicine by contacting the local representative of the marketing authorization holder.
Local representative in Spain
Bausch & Lomb S.A.
Avda. Valdelaparra, nº4
28108 Alcobendas, Madrid
Phone: 91 – 657 63 00
This leaflet was last revised in September 2023.
Detailed information about this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) https://www.aemps.gob.es/
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to MINIMS PREDNISOLONE 5 MG/ML EYE DROPS IN SOLUTION IN SINGLE-DOSE CONTAINERSDosage form: EYEDROP, 10 mg prednisolone acetateActive substance: prednisoloneManufacturer: Abbvie Spain, S.L.U.Prescription requiredDosage form: EYEDROP, 1 mg/mlActive substance: dexamethasoneManufacturer: Pharmaselect International Beteiligungs GmbhPrescription requiredDosage form: EYE DROP, 0.1 %Active substance: dexamethasoneManufacturer: Fidia Farmaceutici S.P.A.Prescription required
Online doctors for MINIMS PREDNISOLONE 5 MG/ML EYE DROPS IN SOLUTION IN SINGLE-DOSE CONTAINERS
Discuss questions about MINIMS PREDNISOLONE 5 MG/ML EYE DROPS IN SOLUTION IN SINGLE-DOSE CONTAINERS, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















