
LYXUMIA 20 micrograms injectable solution

How to use LYXUMIA 20 micrograms injectable solution
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Lyxumia 10micrograms solution for injection
Lyxumia 20micrograms solution for injection
Lixisenatide
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack:
- What is Lyxumia and what is it used for
- What you need to know before you use Lyxumia
- How to use Lyxumia
- Possible side effects
- Storing Lyxumia
- Contents of the pack and other information
1. What is Lyxumia and what is it used for
Lyxumia contains the active substance lixisenatide.
It is an injectable medicine used to help your body control your blood sugar levels when they are too high. It is prescribed for adult patients with type 2 diabetes.
Lyxumia is used together with other diabetes medicines when your blood sugar levels are not adequately controlled with:
- oral anti-diabetics (such as metformin, pioglitazone, sulfonylureas) and/or,
- a basal insulin, a type of insulin that acts throughout the day.
2. What you need to know before you use Lyxumia
Do not use Lyxumia if
- you are allergic to lixisenatide or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Talk to your doctor, pharmacist, or nurse before using Lyxumia if:
- you have type 1 diabetes or "diabetic ketoacidosis" (a complication of diabetes that occurs when your body cannot break down glucose because there is not enough insulin), as this medicine is not suitable for you.
- you have or have ever had pancreatitis (inflammation of the pancreas),
- you have a severe intestinal or gastric problem, such as a disease of the stomach muscles called "gastroparesis" that leads to delayed gastric emptying,
- you have severe kidney disease or are on dialysis, as the use of this medicine is not recommended,
- you are also taking a sulfonylurea or a basal insulin. This is because you may experience low blood sugar (hypoglycemia). Your doctor may want to monitor your blood sugar levels and then decide to reduce the dose of basal insulin or sulfonylurea. Lyxumia should not be used with the combination of both, a basal insulin and a sulfonylurea.
- you are taking other medicines, as there are other medicines such as antibiotics or enteric-coated tablets or capsules that should not stay in the stomach for too long (see section Using Lyxumia with other medicines).
- you experience fluid loss/dehydration; for example, in case of vomiting, nausea, and diarrhea. It is essential that you drink a large amount of fluids to avoid dehydration, especially when starting treatment with Lyxumia.
- you have heart problems that can cause you difficulty breathing or swelling in the ankles, as there is limited experience in this population.
Children and adolescents
There is no experience with Lyxumia in children and adolescents under 18 years, and therefore, the use of Lyxumia is not recommended in this age group.
Using Lyxumia with other medicines
Tell your doctor, pharmacist, or nurse if you are using, have recently used, or might use any other medicines.
The effect of some medicines you take may be affected by Lyxumia. It may be necessary for some medicines, such as antibiotics or enteric-coated tablets or capsules that should not stay in your stomach for too long, to be taken at least one hour before or 4 hours after the injection of Lyxumia.
Pregnancy and breastfeeding
Lyxumia should not be used during pregnancy. It is not known if Lyxumia could harm the fetus.
Lyxumia should not be used during breastfeeding. It is not known if Lyxumia passes into breast milk.
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctor or pharmacist for advice before using this medicine.
Driving and using machines
If you use Lyxumia with a sulfonylurea or a basal insulin, you may experience low blood sugar (hypoglycemia). This could make it difficult to concentrate and you may feel dizzy or drowsy. If this happens, do not drive or use tools or machines.
Important information about some of the ingredients of Lyxumia
This medicine contains less than 1 mmol of sodium (23 mg) per dose, so it is essentially "sodium-free".
This medicine contains metacresol, which may cause allergic reactions.
3. How to use Lyxumia
Follow exactly the administration instructions of this medicine given by your doctor, pharmacist, or nurse. If in doubt, consult your doctor, pharmacist, or nurse again.
How much to inject
- The starting dose is 10 micrograms once a day for the first 14 days (injected using the greenpen).
- From then on, the dose will be 20 micrograms once a day (using the purplepen).
When to inject
Inject Lyxumia within the hour before any meal of the day. It is preferable to inject Lyxumia before the same meal every day, once you have chosen the most suitable meal for your injection.
Where to inject
Inject Lyxumia into the skin (subcutaneously) of your stomach (abdomen), the top of your leg (thigh), or the top of your arm.
Learning to use the pre-filled pens
Before using the pen for the first time, your doctor or nurse will teach you how to inject Lyxumia.
- Always read the "Instructions for Use" that come with the box.
- Always use the pen as indicated in the "Instructions for Use".
Other important information about using the pre-filled pens
You can find more information about using the pens in the "Instructions for Use". The main points are:
- Always use a new needle for each injection. The needle should be discarded after each use in a suitable container for sharp objects, following local recommendations. Talk to your doctor, nurse, or pharmacist about how to dispose of the needles.
- Only use needles compatible with the Lyxumia pen (see "Instructions for Use").
- You must activate the Lyxumia pen before using it for the first time.This is to ensure that it works correctly and that the dose of the first injection is correct.
- If you think the Lyxumia pen may be damaged, do not use it. Get a new one. Do not try to repair the pen.
If you use more Lyxumia than you should
If you use more Lyxumia than you should, call your doctor immediately. Using too much Lyxumia can make you feel nauseous or vomit.
If you forget to use Lyxumia
If you forget a dose of Lyxumia, you can inject it within the hour before your next meal. Do not inject two doses at the same time to make up for a forgotten dose.
If you stop using Lyxumia
Do not stop using Lyxumia without talking to your doctor first. If you stop using Lyxumia, your blood sugar levels may increase.
If you have any other questions about using this medicine, ask your doctor, pharmacist, or nurse.
4. Possible side effects
Like all medicines, Lyxumia can cause side effects, although not everybody gets them.
Rarely, some serious allergic reactions (such as anaphylaxis) have been reported in patients taking Lyxumia. You should seek immediate medical attention if you experience symptoms such as swelling of the face, tongue, or throat that make it difficult to breathe.
Stop using Lyxumia and contact your doctor immediately if you notice any of the following serious side effects:
- Severe and persistent abdominal pain (stomach area) that may radiate to your back, as well as nausea and vomiting, as these may be signs of pancreatitis.
The most frequently reported side effects with Lyxumia that may affect more than 1 in 10 users (frequency: very common) were nausea (feeling sick) and vomiting. These side effects were mostly mild and usually went away over time.
Other side effects
Very common: may affect more than 1 in 10 people
- Diarrhea
- Headache
- Low blood sugar (hypoglycemia) especially when Lyxumia is used with insulin or a sulfonylurea.
The warning signs of low blood sugar can include: cold sweats, cool pale skin, headache, drowsiness, weakness, dizziness, confusion, or irritability, feeling hungry, rapid heartbeat, and nervousness. Your doctor will tell you what to do if you experience low blood sugar.
This is more likely to happen if you are taking a sulfonylurea or a basal insulin at the same time. Your doctor may reduce the dose of these medicines before you start using Lyxumia.
Common: may affect up to 1 in 10 people
- Influenza (flu)
- Upper respiratory tract infection (cold)
- Feeling dizzy
- Indigestion (dyspepsia)
- Back pain
- Cystitis
- Viral infection
- Low blood sugar (when Lyxumia is taken with metformin)
- Drowsiness. Reactions at the injection site (such as itching).
Uncommon: may affect up to 1 in 100 people
- Hives
- Gallstones
- Inflammation of the gallbladder.
Rare: may affect up to 1 in 1,000 people
- Delayed emptying of the stomach.
If you experience side effects, talk to your doctor, pharmacist, or nurse, even if they are not listed in this leaflet.
Reporting side effects
If you experience any side effects, talk to your doctor, pharmacist, or nurse, even if they are not listed in this leaflet. You can also report side effects directly through the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storing Lyxumia
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the label and carton after EXP. The expiry date is the last day of the month stated.
Before first use
Store in a refrigerator (2°C to 8°C). Do not freeze. Keep away from the freezer compartment.
During use of the pen
The pen can be used for 14 days when stored below 30°C. Do not freeze. Do not store with a needle attached. When not using the pen, keep the cap on the pen to protect it from light.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Container Contents and Additional Information
Composition of Lyxumia
- The active ingredient is lixisenatide.
- Lyxumia 10 micrograms solution for injection: Each dose contains 10 micrograms of lixisenatide (50 micrograms per ml).
- Lyxumia 20 micrograms solution for injection: Each dose contains 20 micrograms of lixisenatide (100 micrograms per ml)
- The other ingredients are glycerol 85%, sodium acetate trihydrate, methionine, metacresol, hydrochloric acid (for pH adjustment) and sodium hydroxide solution (for pH adjustment) and water for injections.
Appearance of Lyxumia and Container Contents
Lyxumia is a clear and colorless injectable solution, contained in a glass cartridge inserted into a pre-filled pen.
Each green pen of Lyxumia 10 micrograms solution for injection contains 3 ml of solution containing 14 doses of 10 micrograms. Packaging with 1 pre-filled pen.
Each purple pen of Lyxumia 20 micrograms solution for injection contains 3 ml of solution containing 14 doses of 20 micrograms. Packages with 1, 2 or 6 pre-filled pens. Some pack sizes may only be available in your country.
A starter pack is also available for use during the first 28 days of treatment, containing a green pen of Lyxumia 10 micrograms solution for injection and a purple pen of Lyxumia 20 micrograms solution for injection.
Marketing Authorization Holder
sanofi-aventis groupe
54, rue La Boétie
F – 75008 Paris
France
Manufacturer
Sanofi-Aventis Deutschland GmbH
Industriepark Höchst - 65926 Frankfurt am Main
Germany
You can request more information about this medicine by contacting the local representative of the marketing authorization holder:
Belgium/Belgique/Belgien Sanofi Belgium Tel: +32 (0)2 710 54 00 | Luxembourg/Luxemburg Sanofi Belgium Tel: +32 (0)2 710 54 00 (Belgique/Belgien) |
Bulgaria sanofi-aventis Bulgaria EOOD Tel: +359 (0)2 970 53 00 | Hungary SANOFI-AVENTIS Zrt. Tel: +36 1 505 0050 |
Czech Republic sanofi-aventis, s.r.o. Tel: +420 233 086 111 | Malta Sanofi Malta Ltd. Tel: +356 21493022 |
Denmark sanofi-aventis Denmark A/S Tel: +45 45 16 70 00 | Netherlands sanofi-aventis Netherlands B.V. Tel: +31 (0)182 557 755 |
Germany Sanofi-Aventis Deutschland GmbH Tel: +49 (0)180 2 222010 | Norway sanofi-aventis Norge AS Tel: +47 67 10 71 00 |
Estonia sanofi-aventis Estonia OÜ Tel: +372 627 34 88 | Austria sanofi-aventis GmbH Tel: +43 1 80 185 – 0 |
Greece sanofi-aventis AEBE Tel: +30 210 900 16 00 | Poland Sanofi-aventis Sp. z o.o. Tel: +48 22 280 00 00 |
Spain sanofi-aventis, S.A Tel: +34 93 485 94 00 | Portugal sanofi - Produtos Farmacêuticos, Lda. Tel: +351 21 35 89 400 |
France sanofi-aventis France Tel: 0 800 222 555 Call from abroad: +33 1 57 63 23 23 Croatia sanofi-aventis Croatia d.o.o. Tel: +385 1 600 34 00 | Romania Sanofi Romania SRL Tel: +40 (0) 21 317 31 36 |
Ireland sanofi-aventis Ireland Ltd. T/A Tel: +353 (0) 1 403 56 00 | Slovenia sanofi-aventis d.o.o. Tel: +386 1 560 48 00 |
Iceland Vistor hf. Tel: +354 535 7000 | Slovakia sanofi-aventis Pharma Slovakia s.r.o. Tel: +421 2 33 100 100 |
Italy Sanofi S.p.A. Tel: 800 13 12 12 (technical questions) 800 536 389 (other questions) | Finland Sanofi Oy Tel: +358 (0) 201 200 300 |
Cyprus sanofi-aventis Cyprus Ltd. Tel: +357 22 871600 | Sweden Sanofi AB Tel: +46 (0)8 634 50 00 |
Latvia sanofi-aventis Latvia SIA Tel: +371 67 33 24 51 | United Kingdom Sanofi Tel: +44 (0) 845 372 7101 |
Lithuania UAB « SANOFI-AVENTIS LIETUVA » Tel: +370 5 2755224 |
Date of Last Revision of this Leaflet:
Detailed information on this medicinal product is available on the European Medicines Agency website: http://www.ema.europa.eu.
Lyxumia 20 micrograms solution for injection
lixisenatide
INSTRUCTIONS FOR USE
A pre-filled pen contains 14 doses, and each dose contains 20 micrograms in 0.2 ml.
Section 1 – IMPORTANT INFORMATION
Read these instructions carefully before using your Lyxumia pen.
Keep this leaflet for future reference.
Information about the Lyxumia pen
Lyxumia is a pre-filled pen for injection
- Inject only one dose per day.
Each Lyxumia pen contains 14 pre-selected doses. You do not need to measure each dose.
Talk to your doctor, pharmacist, or nurse about the correct way to inject before using Lyxumia.
If you cannot strictly follow the instructions on your own or are not able to handle the pen (for example, if you have vision problems), use it only if you have help from another person.
About your Lyxumia pen



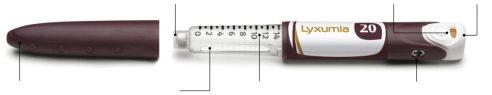






This pen is for single use only. Do not share it with anyone else.
Always check the label to ensure you are using the correct Lyxumia pen. Also, check that it has not passed its expiration date. Using the wrong medicine can harm your health.
Do not attempt to withdraw liquid from the Lyxumia reservoir with a syringe.
About your needle(supplied separately)
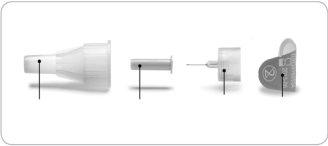



- Use only needles approved for use with Lyxumia. Use disposable pen needles of 29 to 32 gauge with the Lyxumia pen. Ask your doctor, pharmacist, or nurse what gauge and length of needle are most suitable for you.
- If someone else is giving you the injection, they must be careful to avoid needle accidents, as they could transmit an infection.
- Always use a new needle for each injection. This helps prevent contamination of Lyxumia or possible blockage of the needle.
Section 2 – FIRST STEPS
Activate the pen on the same day as your first injection
First, activate your new pen
- Before injecting a dose:before injection, you must remove excess liquid from the new pen. This is done only once and is called the "activation" process. The following steps, 1 to 5, show how to do it.
- Activation is performed to ensure that the pen works correctly and that the dose of the first injection is correct.
- Do not repeatthe activation process, as you will not then get the 14 doses from your Lyxumia pen.
The following images show how the activation window of the injection button of the pen changes after activation.
New pen
(orange window)

Pen ready for injections
(white window)

The pen is activated and ready for injections. The window remains white after activation.
How to activate your new Lyxumia pen
Step 1 Remove the cap and check the pen


Check the liquid. It should be transparent, colorless, and free of visible particles. If it is not, do not use this pen. Contact your doctor, pharmacist, or nurse.
Step 2 Place a needle and remove the caps

Always use a new needlefor activation.
Remove the protective seal from the outer cap of the needle.
Align the needle with the pen. Keep it straight while screwing.

Be careful not to prick yourself when the needle is exposed.
Remove the inner and outer caps of the needle. Keep the outer cap, as you will need it to dispose of the needle later.
Step 3 Pull the injection button out

Pull the injection button firmly until it stops.

Now the arrow will be pointing towards the needle.
Step 4 Press and hold the injection button to remove excess liquid
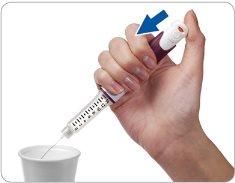
Direct the needle towards a suitable container (such as a glass or paper towel) to collect the liquid and dispose of it.
Press the injection button all the way.You may hear or feel a “click”.
Hold the injection button down and count slowly to 5to extract the last drops.
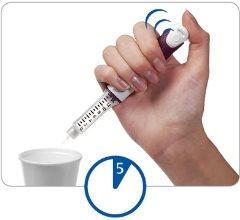

If no liquid comes out, consult the "Questions and Answers" section.
Check that the activation window is now white.
Step 5 The pen is now activated.
Do not activate it again.
It is not necessaryto replace the needle between pen activation and the first injection.
To inject the first dose, go directly to Section 3, step C.
Turn over
Section 3 - DAILY USE OF THE PEN
Go to this section only when the activation window is white.
Inject one dose per day.

Step A. Remove the cap and check the pen

Check the liquid. It should be transparent, colorless, and free of visible particles. If it is not, do not use this pen. If you notice air bubbles, consult the "Questions and Answers" section.
Check the dose number on the pen. It is shown by the position of the black plunger on the dose scale.
Check that the activation window is white. If it is orange, go to Section 2. Check the pen label to ensure you are using the correct medicine.
Step B. Place a new needle and remove the caps

Always use a new needlefor each injection.
Remove the protective seal from the outer cap of the needle.
Align the needle with the pen. Keep it straight while screwing.


Be careful not to prick yourself when the needle is exposed.
Remove the inner and outer caps of the needle. Keep the outer cap, as you will need it to dispose of the needle later.
Step C. Pull the injection button out

Pull the injection button firmly until it stops.

Now the arrow will be pointing towards the needle.
Step D. Press and hold the injection button to inject the dose

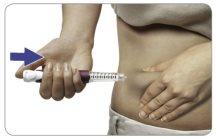
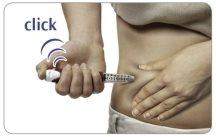


Grasp a skin fold and insert the needle into it (see the "Injection Sites" section to know where to inject).
Press the injection button all the way.You may hear or feel a “click”.
Hold the injection button down and count slowly to 5to get the full dose.
Then the dose will have been administered. Remove the needle from the skin.
Step E Remove and dispose of the needle after each injection

Place the outer cap of the needle on a flat surface. Guide the needle into the outer cap.
Replace the outer cap of the needle.

Squeeze the outer cap to grip the needle and use it to unscrew it from the pen.

Ask your pharmacist how to dispose of the needle that you will not use again. Replace the cap on the pen
Step F. Repeat all the steps of Section 3 for each injection.
Discard the pen 14 days after activation. Do this even if there is still some medicine left in the pen.
Activation and Disposal Table
Write in this table the date you activated your pen and the date you must discard it, 14 days later.
Pen | Activation Date | Disposal Date |
1 | ||
2 | ||
3 | ||
4 | ||
5 | ||
6 |
Storage
General Information
Keep Lyxumia pens in a safe place, out of sight and reach of children.
Protect the Lyxumia pen from dust and dirt.
Replace the pen cap after each use to protect it from light.
Do not use Lyxumia after the expiration date shown on the label and carton. The expiration date is the last day of the indicated month.
Before Activating the Pen:
Store unused Lyxumia pens in the refrigerator (2°C to 8°C).
Do not freeze Lyxumia pens and do not use Lyxumia if it has been frozen.
Allow the pen to reach room temperature before using it.
After Activating the Pen:
Once activated, keep the Lyxumia pen below 30°C. Do not freeze Lyxumia once it has been activated.
Do not store the Lyxumia pen with the needle attached, as this may cause contamination and possible air entry, which could affect the accuracy of the doses.
Once the Lyxumia pen is activated, it can be used for 14 days. Discard the used Lyxumia pen after 14 days. Do this even if there is still some medicine left in the pen.
Disposal
Put the Lyxumia pen cap back on before disposing of it.
- Discard the Lyxumia pen as your pharmacist has told you to dispose of medicines that are no longer needed.
Maintenance
Handle the Lyxumia pen with care.
The outside of the Lyxumia pen can be cleaned with a damp cloth.
Do not get the Lyxumia pen wet, wash it, or put liquid on (lubricate) it, as this could damage it.
If you think the Lyxumia pen may be damaged, do not use it. Do not attempt to repair the pen.
Injection Sites
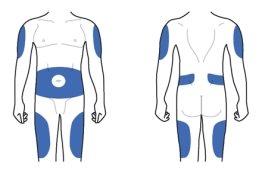
The Lyxumia pen is for injection under the skin, and injections can be performed in any of the areas shown in blue in the previous image. These areas are the thigh, abdominal area, or arm. Consult your doctor, pharmacist, or nurse about the correct injection technique.
Questions and Answers
What if I forget to activate the Lyxumia pen or inject before activation?
If you accidentally inject before activating the pen, do not correct this by administering a second injection. Contact your doctor, pharmacist, or nurse if a blood sugar check is necessary.
What if there are air bubbles in the reservoir?
It is normal to have small air bubbles in the reservoir; they are not harmful to you. Your dose will be correct, and you can continue following the instructions. Contact your doctor, pharmacist, or nurse if you need help.
Why doesn't liquid come out during activation?
The needle may be blocked, or it may not have been screwed on properly. Remove the needle from the pen, attach a new one, and repeat steps 4 and 5. If the liquid still does not come out, the Lyxumia pen may be damaged. Do not use this Lyxumia pen. Ask your doctor, pharmacist, or nurse for advice.
What if it's hard to push the injection button all the way?
The needle may be blocked, or it may not have been screwed on properly. Remove the needle from the skin and from the pen. Insert a new needle and repeat steps D and E. If it's still hard to press the injection button, the Lyxumia pen may be damaged. Do not use this Lyxumia pen. Ask your doctor, pharmacist, or nurse for advice.
If you have any questions about Lyxumia or diabetes, consult your doctor, pharmacist, or nurse, or contact the local representative of the Marketing Authorization Holder listed in this "package leaflet: information for the user" (which is included separately in the box).
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to LYXUMIA 20 micrograms injectable solutionDosage form: INJECTABLE, 10 micrograms + 20 microgramsActive substance: lixisenatideManufacturer: Sanofi Winthrop IndustriePrescription requiredDosage form: INJECTABLE, 10 microgramsActive substance: lixisenatideManufacturer: Sanofi Winthrop IndustriePrescription requiredDosage form: INJECTABLE, 2 mgActive substance: exenatideManufacturer: Astrazeneca AbPrescription required
Online doctors for LYXUMIA 20 micrograms injectable solution
Discuss questions about LYXUMIA 20 micrograms injectable solution, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions











