LOARGYS 5 mg/ml INJECTABLE SOLUTION AND PERFUSION SOLUTION


How to use LOARGYS 5 mg/ml INJECTABLE SOLUTION AND PERFUSION SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Loargys 5 mg/ml Solution for Injection and Infusion
pegzilarginasa
This medicinal product is subject to additional monitoring, which will allow for quick identification of new safety information. You can help by reporting any side effects you may get. The last section of the leaflet includes information on how to report side effects.
Read all of this leaflet carefully before you start taking this medicine, because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or nurse.
- If you experience any side effects, talk to your doctor or nurse, even if they are not listed in this leaflet. See section 4.
Contents of the pack
- What is Loargys and what is it used for
- What you need to know before you take Loargys
- How to take Loargys
- Possible side effects
- Storage of Loargys
- Contents of the pack and further information
- Instructions for use
1. What is Loargys and what is it used for
Loargys contains the active substance pegzilarginasa, which is a modified human enzyme produced by recombinant DNA technology. The medicine is used to treat arginase 1 deficiency (ARG1-D), also known as hyperargininemia, in adults, adolescents, and children from 2 years of age.
Patients with ARG1-D have low levels of an enzyme called arginase. This enzyme helps the body control arginine levels, an amino acid that the body needs to produce proteins. If arginine is not controlled, it can accumulate in the body and cause symptoms, such as problems with muscle control.
Loargys is used in combination with other forms of treating the disease. These may include:
- a low-protein diet
- dietary supplements with essential amino acids
- medications to treat other symptoms of the disease, such as medications that reduce ammonia levels in the body.
How Loargys works
The pegzilarginasa, the active substance of Loargys, works in a similar way to the natural enzyme arginase, which is lacking or not functioning properly in patients with ARG1-D. This reduces arginine levels in the blood, thereby reducing the symptoms of the disease.
2. What you need to know before you take Loargys
Do not take Loargys
- if you have had a severe allergic reaction to pegzilarginasa or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Loargys may cause allergic reactions. This is more likely to happen after the first few doses.
Stop the injection immediately and contact your doctor or the emergency services if you experience any of the following symptoms of a severe allergic reaction: hives, itching all over, chest tightness, difficulty breathing, or low blood pressure. Your doctor may decide that you need additional medical treatment to prevent or treat an allergic reaction.
During your treatment, your doctor will perform blood tests to check what dose of Loargys is suitable for you.
Children and adolescents
The medicine must not be used in children under 2 years of age, as it is not known if Loargys is safe and effective in this age group.
Using Loargys with other medicines
Tell your doctor if you are taking, have recently taken, or might take any other medicines.
Pregnancy, breastfeeding, and fertility
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor before using this medicine. The use of Loargys is not recommended if you are pregnant.
It is not known if the medicine passes into breast milk. If you are breastfeeding, consult your doctor before taking this medicine. Your doctor will help you decide whether to stop breastfeeding or stop the treatment.
Driving and using machines
Loargys has no or negligible influence on the ability to drive and use machines.
Loargys contains sodium and potassium
This medicine contains less than 1 mmol of sodium (23 mg) per dose; this is essentially "sodium-free". This medicine contains potassium, less than 1 mmol (39 mg) per dose, i.e., essentially "potassium-free".
3. How to take Loargys
Loargys will be administered by a healthcare professional. Your doctor will decide the amount of Loargys you will be given.
The recommended initial dose of Loargys is 0.1 mg per kilogram of body weight, administered once a week. Your doctor may increase or decrease the dose to keep arginine levels in the blood under control. Your doctor will prescribe regular blood tests to check your arginine levels in the blood and change your dose if necessary.
Loargys is administered by infusion (drip) directly into a vein or by subcutaneous injection, as considered appropriate by your doctor.
Your doctor may decide that you can be given Loargys at home, in the form of a subcutaneous injection. After receiving training from your doctor or nurse, you may inject Loargys yourself (see instructions in section 7).
Always use this medicine exactly as described in this leaflet or as directed by your doctor, pharmacist, or nurse. If you are unsure, consult your doctor, pharmacist, or nurse.
If you are given more Loargys than you should
Your doctor will make sure that you are given the correct amount of Loargys. If you are given too much Loargys, your arginine level in the blood may become too low. Symptoms may include nausea, vomiting, diarrhea, and fatigue. If you or your doctor suspect that you have been given too much Loargys, you should be closely monitored and receive the necessary treatment.
If you forget to use Loargys
If you have missed a dose of Loargys, contact your doctor to schedule the next dose as soon as possible. You should not be given a double dose to make up for missed doses, and at least 4 days should pass between doses.
If you stop taking Loargys
Your doctor will decide if you should stop taking Loargys. If you stop taking Loargys, it is likely that your arginine level in the blood will increase again.
If you have any other questions about the use of this medicine, ask your doctor or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Very common(may affect more than 1 in 10 people)
- Injection site reaction. Symptoms may include pain, swelling, irritation, redness, and skin rash around the injection site.
- Allergic reaction (hypersensitivity) Symptoms may include swelling of the face, skin rash, and sudden redness of the skin (flushing).
Reporting of side effects
If you experience any side effects, talk to your doctor or nurse, even if they are not listed in this leaflet. You can also report side effects directly through the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Loargys
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the label. The expiry date is the last day of the month stated.
Store in a refrigerator (between 2°C and 8°C). Do not freeze. Keep the container in the outer carton to protect from light.
Once removed from the refrigerator, Loargys can be stored for 2 hours at room temperature not above 25°C.
Medicines should not be disposed of via wastewater or household waste. This will help protect the environment.
6. Container Contents and Additional Information
Composition of Loargys
- The active ingredient is pegzilarginasa.
- Each 0.4 ml vial contains 2 mg of pegzilarginasa.
- Each 1 ml vial contains 5 mg of pegzilarginasa.
- The other ingredients are sodium chloride, potassium dihydrogen phosphate, dipotassium phosphate, glycerol, hydrochloric acid, sodium hydroxide, and water for injections. Loargys contains sodium and potassium (see section 2).
Appearance and Container Contents
Loargys is a colorless to slightly yellow or slightly pink, clear to slightly opalescent liquid in a transparent glass vial.
Each container contains 1 vial with 0.4 ml or 1 ml of solution for injection/infusion.
Only some pack sizes may be marketed.
Marketing Authorization Holder and Manufacturer
Immedica Pharma AB
113 63 Stockholm
Sweden
Manufacturer
Unimedic AB
Storjordenvägen 2
864 31 Matfors
Sweden
Date of Last Revision of this Leaflet
This medicinal product has been authorized under exceptional circumstances. This means that due to the rarity of this disease, it has not been possible to obtain complete information on this medicinal product.
The European Medicines Agency will review any new information that may become available every year and this leaflet will be updated as necessary.
Other Sources of Information
Detailed information on this medicinal product is available on the European Medicines Agency website:. There are also links to other websites on rare diseases and orphan medicines.
This leaflet can be found on the European Medicines Agency website in all EU/EEA languages.
You can also find this leaflet and the approved patient training material on this medicinal product by scanning the QR code below with a smartphone or through the website http://www.loargyspatient.eu

- Instructions for Use
The following steps describe how to prepare and administer Loargys at home, as a subcutaneous injection. If you are going to inject this medicinal product yourself, your doctor or nurse will teach you how to prepare and inject Loargys.
Do not inject this medicinal product yourself unless you have been trained and understand the steps to follow.
Your doctor will prescribe the correct dose and tell you what volume (in ml) to inject. You may need more than one vial to get the correct dose, and you may need to divide the total dose into more than one injection. Your doctor or nurse will tell you exactly what is right for you.
Each vial is for single use; always use a new vial(s) for each dose.
Loargys must not be mixed with other solutions for injection or infusion.
Do not shake.
Preparation:
Make sure you have everything you need for the injection(s):
- Loargys vial(s)
- A graduated syringe
- 1 large needle (e.g., 18 gauge) per vial, for extracting the dose
- 1 small needle (e.g., 26-27 gauge) per injection
- Alcohol swabs
- Gauze pad
- Plaster, if necessary
- Sharps container
|
|
|
|
|
|
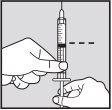
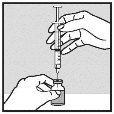
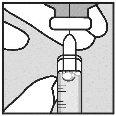
Withdrawal of Solution from Vial:
|
|
|
|
| |
|
|
|
|
| |
| |
| |
Note: If the solution is not to be used immediately, protect the syringe from light. After preparation, Loargys can be stored at room temperature (up to 25°C) for a maximum of 2 hours before administration. After this time, the prepared Loargys can no longer be used and must be discarded. |
Administration of the Dose:
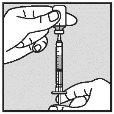
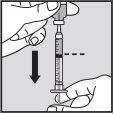
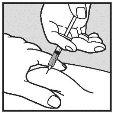
Visually check that the volume contained in the syringe is correct. The volume per injection must not exceed 1 ml. If necessary, multiple injections should be administered in different locations. | |
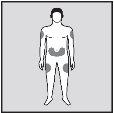
Do not inject into scar tissue or areas that are red, inflamed, or swollen. If injecting into the abdomen, avoid the area around the navel. If more than 1 injection is needed to administer a single dose of Loargys, the injection sites should be at least 3 cm apart. |
|
| |
|
|
|
|
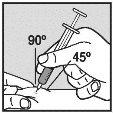
Change the injection site and ensure the new injection site is more than 3 cm away. Slowly push the plunger until you have injected the required volume. Repeat until you have injected your total dose (in ml). Always use a new small needle for each injection.Reminder:if you need to inject a volume greater than 1 ml of Loargys, |
|
| |
|
Keep track of the injection date and all the sites where you have injected; this will help you use a different injection site for the next injection.
--------------------------------------------------------------------------------------------------------------------
This information is intended for healthcare professionals only:
Loargys is indicated for intravenous infusion or subcutaneous injection.
Use aseptic technique when preparing and administering Loargys.
Do not shake.
Instructions for Preparation
- Determine the total volume of Loargys to be administered (and the number of vials needed) based on the patient's weight and dose level.
- Remove the vial(s) from the refrigerator to allow them to reach room temperature.
- Before administration, visually inspect the vial for particles and discoloration.
- Loargys is a colorless to slightly yellow or slightly pink, clear to slightly opalescent liquid, essentially free from visible foreign particles.
- Discard any vial that is not consistent with this appearance.
- Withdraw the planned dose into the syringe.
- The chemical and physical stability of the prepared dose has been demonstrated for 2 hours when stored at room temperature up to 25°C or up to 4 hours if refrigerated between 2°C and 8°C. If the product is not used within these time frames, it must be discarded. From a microbiological point of view, the product must be used immediately after preparation.
For Intravenous Administration
- Dilute with sodium chloride 9 mg/ml (0.9%) solution for injection to obtain the desired volume of infusion (maximum concentration of pegzilarginasa 0.5 mg/ml).
- Administer the intravenous infusion over at least 30 minutes.
- Do not mix other medicinal products with Loargys or infuse other medicinal products simultaneously through the same intravenous access line.
For Subcutaneous Administration
- Administer the solution undiluted by subcutaneous injection in the abdomen, lateral thigh, or lateral or posterior upper arm. Alternate injection sites between doses.
- Do not inject into scar tissue or areas that are red, inflamed, or swollen.
- If injecting into the abdomen, avoid the area around the navel.
- If more than 1 injection is needed to administer a single dose of Loargys, the injection sites should be at least 3 cm apart.
Discard the unused portion of the medicinal product.
No special requirements for disposal.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to LOARGYS 5 mg/ml INJECTABLE SOLUTION AND PERFUSION SOLUTIONDosage form: INJECTABLE INFUSION, 100 UActive substance: laronidaseManufacturer: Sanofi B.V.Prescription requiredDosage form: INJECTABLE PERFUSION, 30 mg/mlActive substance: cerliponase alfaManufacturer: Biomarin International LimitedPrescription requiredDosage form: INJECTABLE INFUSION, UnknownActive substance: imigluceraseManufacturer: Sanofi B.V.Prescription required
Online doctors for LOARGYS 5 mg/ml INJECTABLE SOLUTION AND PERFUSION SOLUTION
Discuss questions about LOARGYS 5 mg/ml INJECTABLE SOLUTION AND PERFUSION SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions