
LIBMELDY 2-10 x 10^6 cells/mL dispersion for perfusion
Ask a doctor about a prescription for LIBMELDY 2-10 x 10^6 cells/mL dispersion for perfusion

How to use LIBMELDY 2-10 x 10^6 cells/mL dispersion for perfusion
Introduction
Package Leaflet: Information for the Patient or Carer
Libmeldy 2-10 x 106cells/ml dispersion for infusion
atidarsagene autotemcel
This medicine is subject to additional monitoring, which will help to quickly identify new safety information. You can contribute by reporting any side effects your child may have. The last part of section 4 will include information on how to report side effects.
Read all of this leaflet carefully before your child is given this medicine, because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your child's doctor or nurse.
- If your child gets any side effects, talk to your child's doctor or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
- Your child's doctor or nurse will give you a patient information card that contains important safety information that you need to know about your child's treatment with Libmeldy. Read the patient information card carefully and follow the instructions it contains.
- You must carry the patient information card with you at all times and show it to your child's doctor or nurse when you see them or if your child is admitted to hospital.
Contents of the pack
- What is Libmeldy and what is it used for
- What you need to know before your child is given Libmeldy
- How Libmeldy is prepared and administered
- Possible side effects
Side effects of the conditioning medicine
Side effects of Libmeldy
- Storage of Libmeldy
- Contents of the pack and further information
1. What is Libmeldy and what is it used for
What is Libmeldy
Libmeldy is a type of medicine called gene therapy. It is made especially for your child from their own bone marrow or blood cells.
What Libmeldy is used for
Libmeldy is used to treat a serious disease called metachromatic leukodystrophy (MLD):
- in children with the «late infantile» or «early juvenile» forms of the disease who have not yet developed any signs or symptoms;
- in children with the «early juvenile» form of the disease who have started to develop symptoms, but whose symptoms are not worsening rapidly.
People with MLD have a fault in the gene for producing an enzyme called arylsulfatase A (ARSA). This leads to a build-up of substances called sulfates in the brain and nervous system, causing damage to the nervous system and a progressive loss of physical and, later, mental abilities, ultimately leading to death.
How Libmeldy works
Cells called stem cells are taken from your child's bone marrow or blood. These cells are then modified in a laboratory to add a working gene for ARSA. When your child is given Libmeldy, which is made up of these modified cells, the cells will start to produce ARSA to break down the sulfates in your child's nerve cells and other cells in their body. This process is expected to slow down the progression of the disease and improve your child's quality of life.
Libmeldy is given by drip (infusion) into a vein (intravenously). For more information on what happens before and during treatment, see section 3, How Libmeldy is administered.
If you have any questions about how Libmeldy works or why it has been prescribed for your child, ask your child's doctor.
2. What you need to know before your child is given Libmeldy
Libmeldy must not be given to your child:
- If your child is allergic to any of the components of this medicine (listed in section 6). If you think your child may be allergic, ask their doctor.
- If your child has previously undergone gene therapy with their own blood stem cells.
- If your child is allergic to or if their doctor thinks your child may experience unacceptable side effects to any of the ingredients of the medicines your child will receive before treatment with Libmeldy (see section 3).
Warnings and precautions
- Information on cell-based medicines like Libmeldy will be kept for 30 years in the hospital. The information that will be kept about your child will be their name and the batch number of Libmeldy they have received.
- Libmeldy is made from your child's own stem cells and must only be given to your child.
Before treatment with Libmeldy
- Your child will be assessed by their doctor to confirm they have MLD and to assess the symptoms and effects of their disease before the decision to use Libmeldy is made. It is possible that your child may not show any physical signs of the disease at the time of the initial assessment.
If your child's MLD has progressed and worsened before the start of treatment, their doctor may decide that their disease has reached a «rapidly progressive phase». If this happens, it may be that your child will not benefit from treatment and their doctor may decide not to give them Libmeldy.
- Your child may be given medicines known as mobilisation medicinesand conditioning medicines(see sections 3 and 4 for more information on these medicines, including possible side effects).
- Central venous catheters are thin, flexible tubes that the doctor will insert into a large vein to access your child's bloodstream. The risks of these lines are infections and the formation of blood clots. The doctor and nurses will monitor your child for any complications of the central venous catheter.
- Libmeldy has been tested for the presence of infectious microorganisms before it is given to your child. There is a small risk of infection. The doctors and nurses will monitor your child during the infusion for signs of infection and will provide treatment if necessary.
- The doctor will check your child's thyroid gland. The thyroid gland is in the neck and produces hormones that are important to help the body work normally. It will also be monitored after treatment if necessary.
After treatment with Libmeldy
- After treatment, your child may be asked to take part in a follow-up studyfor up to 15 years to better understand the long-term effects of Libmeldy.
- If your child needs a blood transfusion within the first three months after receiving Libmeldy, the blood products must be irradiated. This means that the white blood cells, called lymphocytes, have been reduced to minimize the risk of a transfusion reaction. The doctor will monitor your child for any transfusion accident.
- Your child's red blood cells will be low for some time after treatment with Libmeldy. This affects the blood cells that fight infections, called neutrophils, which can be measured with a simple blood test. If your child's neutrophils remain low after 60 days, this may be called «graft failure». In this case, your child's doctor may decide to give them the previously collected rescue cells (see section 3). These rescue cells have not had the working ARSA gene added and will not produce this enzyme.
- After receiving the conditioning medicine, your child may have a low number of platelets in their blood. This means that their blood may not be able to clot normally and your child may be prone to bleeding for some time after treatment. The doctor will monitor your child's platelet count with blood tests and will provide treatment if necessary. This may include a platelet transfusion to help increase their platelet count.
- A metabolic acidosis may occur. This is a condition where the level of acid in the blood increases. There may be many different reasons for this, and it is more common in patients with MLD. The symptoms of metabolic acidosis include shortness of breath, rapid breathing, nausea (feeling sick) and vomiting. The doctor will monitor your child for signs and symptoms of metabolic acidosis.
- The insertion of a new gene into the stem cells could, in theory, cause cancer in the blood (leukaemia and lymphoma). After treatment, your child's doctor will monitor them for any signs of leukaemia or lymphoma.
- During clinical studies, some patients developed antibodies against the ARSA enzyme, called anti-ARSA antibodies (see side effects of Libmeldy in section 4). This resolved on its own or after treatment with adapted medicines. Your child's doctor will test their blood for anti-ARSA antibodies and will provide treatment if necessary.
- After your child has received Libmeldy, they will be monitored with regular blood tests. This will include measuring antibodies, known as immunoglobulins, in their blood. If the level is low, your child may need replacement treatment with immunoglobulin. Your child's doctor will talk to you if this is necessary.
- Libmeldy is made using parts of the human immunodeficiency virus (HIV), which have been altered so that they cannot cause an infection. The altered virus is used to insert the ARSA gene into your child's stem cells. Although this medicine will not infect your child with HIV, having Libmeldy in their blood may cause a false positive result in an HIV test with some commercial tests (the so-called «PCR-based tests») that detect a part of the HIV used to make Libmeldy. If your child's HIV test result is positive after treatment with Libmeldy, contact their doctor or nurse.
- After treatment with Libmeldy, your child will not be able to donate blood, organs, tissues or cells. This is because Libmeldy is a gene therapy product.
Before Libmeldy is given to your child, the doctor:
- Will check your child's lungs, heart, kidneys, liver and blood pressure.
- Will look for signs of infection; any infection will be treated before your child receives Libmeldy.
- Will check for hepatitis B, hepatitis C, human T-cell leukaemia virus (HTLV), HIV or mycoplasma infection.
- Will check if your child has been vaccinated in the last six weeks or if a vaccination is planned in the next few months.
When Libmeldy treatment cannot be completed
Before Libmeldy is given to your child, they will receive a conditioning medicine to remove the cells from their bone marrow.
If Libmeldy cannot be given after your child has received the conditioning medicine, or if the modified stem cells do not engraft (take) in your child's body, the doctor may decide to give them the previously collected rescue cells by infusion (see also section 3, How Libmeldy is administered). These rescue cells have not had the working ARSA gene added and will not produce this enzyme. For more information, contact your child's doctor.
Other medicines and Libmeldy
Tell your doctorif your child is taking, has recently taken or might take any other medicines, including those that can be bought without a prescription.
- Your child must not take any medicine for HIV infection from at least one month before they are given the mobilisation medicines or have a bone marrow sample taken, until at least seven days after the infusion with Libmeldy (see also section 3, How Libmeldy is prepared and administered).
- Your child must not be given vaccines called live attenuated vaccinesfor six weeks before they are given the conditioning medicine to prepare them for Libmeldy treatment, or after treatment while their immune system is recovering.
Driving and using machines
Libmeldy has no influence on the ability to drive or use machines. However, the mobilisation and conditioning medicines may cause dizziness and fatigue.
Libmeldy contains sodium and dimethyl sulfoxide
This medicine contains 35-560 mg of sodium (main component of common/table salt) per dose. This is equivalent to 2-28% of the maximum recommended daily intake of sodium for an adult.
If your child has not been exposed to dimethyl sulfoxide (a substance used to preserve frozen cells) before, the doctor or nurse must monitor your child for any reaction during the infusion and at each hour for the next three hours after the infusion.
3. How Libmeldy is prepared and administered
Because Libmeldy is made from your child's own stem cells, their bone marrow or blood will be taken about two months before treatment to prepare the medicine. The bone marrow can be taken from your child's hip bones and the blood can be taken from their veins. For more information, ask your child's doctor.
If the stem cells are taken from your child's bone marrow:
- Your child will be given medicine to help them relax or to make them unconscious before the procedure. The doctor will take the bone marrow from your child using a special syringe.
If the stem cells are taken from your child's blood:
- Your child will first be given a mobilisation medicine to move the blood stem cells from their bone marrow into their bloodstream.
- Then, the blood stem cells can be taken using a machine that separates the components of the blood (apheresis machine). It may take more than one day to collect enough stem cells from the blood to make Libmeldy.
The stem cells taken from the bone marrow or blood will be divided into:
- The backup sample, which will be frozen and stored, and will be given to your child as replacement stem cells if Libmeldy cannot be given or does not work (see «When Libmeldy treatment cannot be completed» in section 2).
- The treatment sample, which will be sent to prepare Libmeldy, by adding a working copy of the ARSA gene to the stem cells in the sample.
How Libmeldy is administered to your child
- Libmeldy will be given to your child in a specialized treatment centre by doctors who are specialized in the use of this type of medicine.
- The doctors will check that the infusion bags of Libmeldy are labelled as being made from your child's own sample.
- Libmeldy is a one-time treatment and cannot be given to your child again in the future.
When | What happens | Why |
About two months before Libmeldy infusion | Mobilisation medicine is given if Libmeldy is made from blood stem cells | To move the blood stem cells from your child's bone marrow into their bloodstream. |
About two months before Libmeldy infusion | Bone marrow or blood is taken | To make Libmeldy and to serve as backup cells if needed. |
Five days before Libmeldy infusion | Conditioning medicine is given for 3-4 days in hospital | To prepare your child's bone marrow for treatment by destroying the cells in the bone marrow so that they can be replaced by the modified Libmeldy cells. |
15-30 minutes before Libmeldy infusion | An antihistamine medicine may be given | To help prevent an allergic reaction to the infusion. |
Start of Libmeldy infusion | Libmeldy is given by drip (infusion) into a vein. This will be done in a | To add stem cells with the ARSA gene to your child's bone marrow. |
hospital and will take less than 30 minutes for each infusion bag. The number of bags will vary depending on the patient. | ||
After Libmeldy infusion | Your child will stay in hospital for about 4-12 weeks | To recover and to be monitored to check if the treatment is working and to take action if any side effects occur until the doctor thinks it is safe for your child to leave hospital. |
4. Possible Adverse Effects
Like all medicines, this medicine can cause adverse effects, although not all people suffer from them.
Some adverse effects are related to the conditioning medicine used to prepare your child's bone marrow for treatment with Libmeldy.
Talk to your child's doctor about the adverse effects of the conditioning medicine.
You can also read the package inserts of that medicine.
Adverse Effects of the Conditioning Medicine
Inform your doctor or nurse immediatelyif your child suffers from any of the following adverse effects after receiving the conditioning medicine. They usually appear between the first few days and several weeks after receiving the conditioning medicine, but they can also develop much later.
Very Common Adverse Effects (may affect more than 1 in 10 people)
- blood test showing low levels of white blood cells with or without fever
- metabolic acidosis, a condition in which acid levels in the blood are elevated
- inflammation and sores in the mouth and lips
- discomfort (vomiting)
- hepatomegaly
- pain in the upper right part of the abdomen (belly) below the ribs, yellowing of the eyes or skin, rapid weight gain, swelling of the arms, legs, and abdomen, and difficulty breathing. These may be signs of a serious liver disease called veno-occlusive disease
- loss or decrease of ovarian function
Common Adverse Effects (may affect up to 1 in 10 people)
- abnormal bleeding or bruising: may be caused by low platelet levels, which reduce the blood's ability to clot
- infections that may cause your child to feel hot (fever), cold, or sweaty
- infection in the chest (pneumonia)
- infection of the organs involved in urinating (such as the bladder and urinary tract)
- low red blood cell count (anemia)
- excess fluid in the body
- fluid accumulation in the abdomen
- sleeping problems
- headache
- nasal bleeding
- pain in the mouth and throat
- diarrhea
- bleeding in the intestinal tract
- discomfort (nausea)
- increased liver enzymes (transaminases and aminotransferases) observed in blood tests
- itching
- back pain
- bone pain
- decreased urine production
- fever
- positive Aspergillustest (lung disease caused by a fungus)
Adverse Effects of Libmeldy
The following adverse effects have been reported with Libmeldy.
Very Common Adverse Effects (may affect more than 1 in 10 people)
- positive test for antibodies against ARSA. Antibodies are the body's natural defense against anything the body considers foreign.
Reporting Adverse Effects
If your child experiences adverse effects, consult your child's doctor or nurse, even if they are adverse effects that do not appear in this package insert. You can also report them directly through the national reporting system included in Appendix V. By reporting adverse effects, you can contribute to providing more information on the safety of this medicine.
5. Storage of Libmeldy
This information is intended only for healthcare professionals.
Since this medicine will be administered in a hospital, the hospital will be responsible for the correct storage of the medicine before and during its use, as well as its correct disposal.
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiration date shown on the outer packaging and the labels of the infusion bag.
Do not use this medicine if the infusion bag is damaged or leaking.
Store at <-130 ºC for up to six months. Do not thaw the medicine until it is ready to be used. Once thawed, store at room temperature (20 ºC-25 ºC) and use within two hours. Do not re-freeze.
This medicine contains genetically modified human cells. The unused medicine or waste material must be disposed of in accordance with local guidelines for handling human-derived materials.
6. Package Contents and Additional Information
Composition of Libmeldy
The active ingredient of Libmeldy consists of your child's own stem cells containing functional copies of the ARSA gene. The concentration per bag is 2-10 × 10^6 cells per milliliter.
The other ingredients are a solution used to preserve the frozen cells and sodium chloride (see section 2, Libmeldy contains sodium).
Appearance of Libmeldy and Package Contents
Libmeldy is a clear to slightly turbid cell dispersion, colorless to yellow or pink, supplied in one or more transparent infusion bags, each packaged in a bag within a closed container.
Your child's name and date of birth, as well as the coded information that identifies your child as a patient, are printed on each infusion bag and on each container.
Marketing Authorization Holder
Orchard Therapeutics (Netherlands) B.V.
Basisweg 10,
1043AP Amsterdam,
Netherlands
Manufacturer
AGC Biologics S.p.A.
Zambon Scientific Park
Via Meucci 3
20091 Bresso (MI)
Italy
AGC Biologics S.p.A.
Via Olgettina 58
20132
Milan
Italy
Date of Last Revision of this Package Insert:
Other Sources of Information
Detailed information on this medicine is available on the European Medicines Agency website: http://www.ema.europa.eu.
< -------------------------------------------------------------------------- >
This information is intended only for healthcare professionals:
It is essential that you read the entire content of this procedure before administering Libmeldy.
Precautions to be taken before handling or administering the medicine
- This medicine contains genetically modified human red blood cells. Healthcare professionals handling Libmeldy must take adequate precautions (wear gloves, protective clothing, and eye protection) to avoid possible transmission of infectious diseases.
- Libmeldy must remain at <-130 ºC at all times until the contents of the bag are thawed for infusion.
Definition of the administered dose
- The dose to be infused and the number of infusion bags of Libmeldy to be used must be defined according to the total number of CD34+ cells supplied, as indicated on the batch information label (i.e., the "supplied dose", calculated according to the patient's weight at the time of cell extraction). The dose of Libmeldy to be administered must also take into account the patient's weight at the time of treatment and the fact that any bag used must be administered in its entirety.
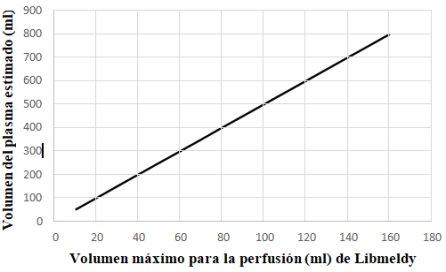
- Special consideration should be given to the infusion volume in relation to the patient's age and weight. When the Libmeldy infusion dose is to be more than one bag, it must be ensured before infusion that the volume of the medicine to be infused is compatible with the recommended limit, i.e., the total volume of dimethyl sulfoxide administered must be <1% of the patient's estimated plasma volume. Therefore, the maximum volume of Libmeldy administered must be <20% of the patient's estimated plasma volume.
- The following graph is provided as a reference to determine the maximum volume of Libmeldy that can be infused to a patient based on their estimated plasma volume.
Guideline on the safety limit of dimethyl sulfoxide: the maximum volume of Libmeldy administration must be < 20% of the patient's estimated plasma volume.
Preparation for infusion
- Several infusion bags may be used for each patient. Each infusion bag is provided within a complete overwrap bag, which is inside a metal carton.
- The overwrap bag(s) must be kept inside the metal carton(s) in the liquid nitrogen vapor phase at <-130 ºC until they are ready for thawing and infusion.
- Count all infusion bags and confirm that each infusion bag is within the expiration date shown on the attached batch information label.
- A sterile sodium chloride 9 mg/ml (0.9%) injectable solution should be available to prime the tubing before infusion and to flush the bag and tubing after infusion.
Check before thawing
- Do not remove the metal carton from cryogenic storage or thaw Libmeldy until the patient is ready for infusion. The timing of thawing of the infusion bag(s) containing Libmeldy and infusion should be coordinated. Confirm the infusion time in advance and adjust the start time with thawing so that Libmeldy is available for infusion when the recipient is ready.
- Open the metal carton and inspect the complete overwrap bag and infusion bag for any loss of integrity before thawing. If an infusion bag is affected, follow local guidelines for handling human-derived waste materials and contact Orchard Therapeutics immediately.
- Before thawing Libmeldy, verify that the patient's identity matches the unique patient information shown on the labels of the packages and on the batch information label included. Libmeldy is intended for autologous use only. Do not thaw or infuse Libmeldy if the patient-specific label information on the infusion bag does not match the patient.
Thawing
- After carefully removing it from the metal carton, thaw the infusion bag in its complete overwrap bag at 37 ºC in a controlled thawing material until no visible ice is present in the infusion bag.
- Once thawing is complete, the bag must be immediately removed from the thawing material.
- The complete overwrap bag should be carefully opened to remove the infusion bag, which should be kept at room temperature (20 ºC-25 ºC) until infusion.
- Gently massage the infusion bag to resuspend the cells. The contents of the infusion bag should be inspected for any remaining visible cell aggregates. Small cell clusters should be dispersed with gentle manual mixing. Do not shake the bag.
- The infusion bag must not be washed, centrifuged, sampled, or resuspended in other media before infusion.
- Libmeldy must not be irradiated, as irradiation could inactivate the product.
- If more than one infusion bag is provided for the patient's treatment dose, the next bag should only be thawed after the contents of the previous bag have been completely infused.
Administration
- Libmeldy must be administered as an intravenous infusion through a central venous catheter, in accordance with the standard procedures of the specialized treatment center for the administration of cell therapy products.
- The recommended administration equipment consists of a blood transfusion set equipped with a 200 µm filter.
- Each bag should be infused by gravity within two hours of thawing, including any interruption during infusion, to maintain the maximum viability of the product.
- The maximum infusion rate is 5 ml/kg/h, and the contents of each bag should be infused in approximately 30 minutes.
- When more than one bag of Libmeldy is needed, only one bag of the product should be infused per hour.
- Patient monitoring is recommended, especially for patients who have not been previously exposed to dimethyl sulfoxide. Vital signs (blood pressure, heart rate, and oxygen saturation) and the appearance of any symptoms should be monitored for up to three hours after infusion.
- At the end of the infusion, rinse the remaining Libmeldy in the infusion bag and any tubing used with the 9 mg/ml (0.9%) sodium chloride injectable solution to ensure that the maximum amount of cells is infused to the patient. Special consideration should be given to the infusion volume in relation to the patient's age and weight.
Precautions to be taken in the disposal of the medicine
- Libmeldy contains genetically modified human cells. Local guidelines for handling human-derived materials should be followed for unused medicine or waste material.
- All material that has come into contact with Libmeldy (solid and liquid waste) should be handled and disposed of as potentially infectious waste, in accordance with local guidelines for handling human-derived materials.
Accidental exposure
- Accidental exposure to Libmeldy should be avoided. In case of accidental exposure, local guidelines for handling human-derived materials should be followed, which may include washing the contaminated skin and disposing of contaminated clothing. Work surfaces and materials that may have come into contact with Libmeldy should be decontaminated with a suitable disinfectant.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to LIBMELDY 2-10 x 10^6 cells/mL dispersion for perfusionDosage form: INJECTABLE INFUSION, 100 UActive substance: laronidaseManufacturer: Sanofi B.V.Prescription requiredDosage form: INJECTABLE PERFUSION, 30 mg/mlActive substance: cerliponase alfaManufacturer: Biomarin International LimitedPrescription requiredDosage form: INJECTABLE INFUSION, UnknownActive substance: imigluceraseManufacturer: Sanofi B.V.Prescription required
Online doctors for LIBMELDY 2-10 x 10^6 cells/mL dispersion for perfusion
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for LIBMELDY 2-10 x 10^6 cells/mL dispersion for perfusion – subject to medical assessment and local rules.














