
LEVOBUPIVACAINE KABI 5 mg/ml INJECTABLE SOLUTION AND PERFUSION SOLUTION

How to use LEVOBUPIVACAINE KABI 5 mg/ml INJECTABLE SOLUTION AND PERFUSION SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Levobupivacaine Kabi 5 mg/ml Solution for Injection and Infusion EFG
Levobupivacaine hydrochloride
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or nurse.
- If you get any side effects, talk to your doctor or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack:
- What is Levobupivacaine Kabi and what is it used for
- What you need to know before you are given Levobupivacaine Kabi
- How Levobupivacaine Kabi will be given to you
- Possible side effects
- Storage of Levobupivacaine Kabi
- Contents of the pack and other information
1. What is Levobupivacaine Kabi and what is it used for
Levobupivacaine Kabi belongs to a group of medicines called local anaesthetics. This type of medicine is used to numb parts of the body or to relieve pain.
In adults and adolescents over 12 years:
Levobupivacaine Kabi is used as a local anaesthetic to numb parts of the body before major surgery (e.g. epidural for caesarean section) and minor surgery (e.g. eye or mouth).
It is also used for pain relief
- after major surgery
- during childbirth
In children (under 12 years):
Levobupivacaine Kabi can also be used in children under 12 years to numb parts of the body before an operation and for pain relief after minor surgery, such as an inguinal hernia.
Levobupivacaine Kabi has not been tested in children under 6 months.
2. What you need to know before you are given Levobupivacaine Kabi
Do not use Levobupivacaine Kabi:
- if you are allergic (hypersensitive) to levobupivacaine, to any other local anaesthetic or to any of the other ingredients of this medicine (listed in section 6).
- if you have very low blood pressure.
- to numb a part of the body by injecting Levobupivacaine Kabi into a vein.
- to relieve pain by injecting it into the area around the neck of the womb (cervix) during the early stages of labour (paracervical block).
Warnings and precautions
Tell your doctor before you are given Levobupivacaine Kabi if you have any of the following conditions or diseases. You may need closer monitoring or a lower dose.
- if you have any heart disease
- if you have a disease of the nervous system
- if you are weak or ill
- if you are elderly
- if you have liver disease.
Using Levobupivacaine Kabi with other medicines
Tell your doctor or nurse if you are taking, have recently taken or might take any other medicines. Tell them especially if you are taking medicines for:
- irregular heartbeats (such as mexiletine)
- fungal infections (such as ketoconazole) as they may affect the metabolism of Levobupivacaine Kabi.
- asthma (such as theophylline) as they may affect how long Levobupivacaine Kabi stays in your body.
Pregnancy and breast-feeding
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor for advice before using this medicine.
Levobupivacaine Kabi should not be given to relieve pain by injecting it into the area around the neck of the womb or cervix during labour (paracervical block).
The effects of Levobupivacaine Kabi on the fetus during the first stages of pregnancy are not known. Therefore, Levobupivacaine Kabi should not be used during the first three months of pregnancy unless your doctor considers it necessary.
It is not known whether levobupivacaine passes into breast milk. However, based on experience with similar drugs, it is expected that only small amounts of levobupivacaine will pass into breast milk. Breast-feeding is therefore possible after using a local anaesthetic.
Driving and using machines
Using Levobupivacaine Kabi may have a considerable effect on your ability to drive and use machines. Do not drive or use machines until the effects of Levobupivacaine Kabi and the effects of the surgery have passed. Before you leave the hospital, ask your doctor or nurse if you can drive or use machines.
Levobupivacaine Kabi contains sodium
This medicine contains 3.6 mg of sodium (a major component of cooking/table salt) in each ml. This is equivalent to 0.18% of the maximum recommended daily intake of sodium for an adult.
3. How Levobupivacaine Kabi will be given to you
Your doctor will give you Levobupivacaine Kabi by injection through a needle or through a small tube inserted into your back (epidural). Levobupivacaine Kabi can also be injected into other parts of the body to numb the area to be treated, such as the eye, arm or leg.
Your doctor and nurse will carefully monitor you while you are being given Levobupivacaine Kabi.
Dose
The amount of Levobupivacaine Kabi that you will be given and how often it will be given will depend on what it is being used for and your physical condition, age and weight. You will be given the lowest dose that achieves the numbness in the required area. The dose will be carefully calculated by your doctor.
When Levobupivacaine Kabi is used to relieve pain during childbirth or for a caesarean section (by epidural), the dose given will be carefully controlled.
If you are given too much Levobupivacaine Kabi
If you are given too much Levobupivacaine Kabi, you may experience numbness of the tongue, dizziness, blurred vision, muscle twitching, severe difficulty breathing (including breathing stop) and even convulsions. If you notice any of these symptoms, tell your doctor immediately. In some cases, too much Levobupivacaine Kabi may also cause low blood pressure, slow heart rate or fast heart rate and changes in your heart rhythm. Your doctor may give you other medicines to help stop these symptoms.
If you have any other questions about the use of this medicine, ask your doctor or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Tell your doctor or nurse immediately if you think you have any of the following side effects. Some of the side effects of Levobupivacaine Kabi can be serious.
Very common(may affect more than 1 in 10 people):
- feeling tired or weak, difficulty breathing, paleness (these are all signs of anaemia)
- low blood pressure
- nausea
Common(may affect up to 1 in 10 people):
- dizziness
- headache
- vomiting
- fetal distress
- back pain
- fever
- post-operative pain
Frequency not known(frequency cannot be estimated from the available data):
- severe allergic reactions (hypersensitivity) causing severe difficulty breathing, difficulty swallowing, hives and very low blood pressure
- allergic reactions (hypersensitivity) recognised by having red and irritated skin, sneezing, excessive sweating, increased heart rate, fainting or swelling of the face, lips, mouth, tongue or throat
- loss of consciousness
- drowsiness
- blurred vision
- breathing stop
- heart block or cardiac arrest
- localised tingling
- numb tongue
- muscle weakness or tremors
- loss of control of urine and faeces
- paralysis
- convulsions
- tingling, numbness or other strange sensation
- prolonged erection of the penis, which can be painful
- nervous system disorders which may include drooping eyelids, small pupils (the black centre of the eye), sunken eye socket, sweating and/or flushing of one side of the face.
Bradycardia, tachycardia and changes in heart rhythm which may be seen on an ECG (electrocardiogram) have also been reported as side effects.
In very rare cases, some side effects may occur long-term or become permanent.
Reporting of side effects
If you get any side effects, talk to your doctor or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Spanish Medicines Surveillance System for Human Use: https://www.notificaram.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Levobupivacaine Kabi
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the carton after EXP. The expiry date refers to the last day of the month shown.
Your doctor will store this medicine for you.
The solution should be used immediately after opening.
Do not use this medicine if you notice particles in the solution.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help protect the environment.
6. Contents of the pack and other information
Composition of Levobupivacaine Kabi
- The active substance is levobupivacaine (as hydrochloride)
Levobupivacaine Kabi 5 mg/ml solution for injection and infusion: 1 ml of solution contains 5 mg of levobupivacaine (as levobupivacaine hydrochloride). Each 10 ml ampoule contains 50 mg of levobupivacaine as levobupivacaine hydrochloride.
- The other ingredients are water for injections, sodium chloride and a small amount of sodium hydroxide and hydrochloric acid.
This medicine contains an excipient with a known effect (sodium). See section 2 for further information.
pH: 4.0 – 6.0
Osmolality: 271 – 372 mOsmol/l
Appearance of the product and pack contents
This medicine is a clear, colourless solution in polypropylene ampoules in sterile blister packs. Each ampoule contains 10 ml of solution. It is supplied in packs of 5, 10 or 20 ampoules.
Not all pack sizes may be marketed.
Marketing Authorisation Holder and Manufacturer
Marketing Authorisation Holder:
Fresenius Kabi España S.A.U
C/ Marina 16-18,
08005-BARCELONA
Manufacturer:
HP Halden Pharma AS
Svinesundsveien 80
1788 Halden
Norway
This medicine is authorised in the Member States of the European Economic Area under the following names:
Member State | Marketing Authorisation Name |
Belgium | Levobupivacaïne Fresenius Kabi 5 mg/ml oplossing voor injectie/infusie |
Croatia | Levobupivacaine Kabi 5 mg/ml otopina za injekciju/infuziju |
Czech Republic | Levobupivacaine Kabi 5 mg/ml |
France | Levobupivacaïne Kabi 5 mg/ml, solution injectable/ pour perfusion |
Ireland | Levobupivacaine 5 mg/ml solution for injection/infusion |
Italy | Levobupivacaina Kabi |
Netherlands | Levobupivacaïne Fresenius Kabi 5 mg/ml oplossing voor injectie/infusie |
Portugal | Levobupivacaína Kabi |
Slovenia | Levobupivakain Kabi 5 mg/ml raztopina za injiciranje/infundiranje |
Slovakia | Levobupivacaine Kabi 5 mg/ml |
Spain | Levobupivacaína Kabi 5 mg/ml solución inyectable y para perfusión |
United Kingdom | Levobupivacaine 5 mg/ml solution for injection/infusion |
Date of last revision of this leaflet:October 2018
Detailed and up-to-date information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
-------------------------------------------------------------------------------------------------------------------------
This information is intended only for healthcare professionals:
Levobupivacaine Kabi5 mg/ml solution for injection and infusion EFG
Instructions for use / handling
Levobupivacaine Kabi 5 mg/ml solution for injection and infusion is for single use only. Discard any unused solution. Use only if the pack is intact.
The solution/dilution should be inspected visually before use. Only clear solutions without visible particles should be used.
A sterile blister pack should be chosen when a sterile ampoule surface is required. The ampoule surface is not sterile if the blister pack is damaged.
Dilutions of standard solutions of levobupivacaine should be made with sodium chloride 9 mg/ml (0.9%) injection solution using aseptic techniques.
It has been shown that 8.4 µg/ml of clonidine, 50 µg/ml of morphine and 2 - 4 µg/ml of fentanyl and 0.4 µg/ml of sufentanil are compatible with levobupivacaine in a sodium chloride 9 mg/ml (0.9%) injection solution.
Shelf-life after first opening: The product should be used immediately.
Shelf-life after dilution:
The chemical and physical stability of Levobupivacaine Kabi diluted with 9 mg/ml sodium chloride (0.9%) to a final concentration of 0.625 mg/ml and 1.25 mg/ml, respectively, has been demonstrated for 30 days at 2-8°C or 20-25°C.
The chemical and physical stability of Levobupivacaine Kabi diluted with 9 mg/ml sodium chloride (0.9%) to a final concentration of 0.625 mg/ml and 1.25 mg/ml, respectively,
- with 8.4 µg/ml of clonidine hydrochloride, 50 µg/ml of morphine sulphate and 2 - 4 µg/ml of fentanyl citrate for 30 days at 2-8°C or 20-22°C.
- with sufentanil added to a concentration of 0.4 µg/ml for 30 days at 2-8°C or for 7 days at 20-22°C.
From a microbiological point of view, the product should be used immediately. If not used immediately, the in-use storage times and conditions are the responsibility of the user and would normally not be longer than 24 hours at 2-8°C, unless the dilution and mixing have been prepared in controlled and validated aseptic conditions.
Levobupivacaine Kabi should not be mixed with other medicines except those mentioned above. Dissolving with alkaline solutions such as sodium bicarbonate may cause precipitation.
Experience with the safety of levobupivacaine treatment for more than 24 hours is limited.
Method of administration
Administration of Levobupivacaine Kabi should only be performed by a doctor who has the necessary training and experience or under their supervision.
For information on dosage, see the Summary of Product Characteristics.
Aspiration should be carefully performed before and during injection to prevent intravascular injection.
Aspiration should be repeated before and during administration of a bolus dose, which should be injected slowly and in increments of dose at a rate of 7.5 - 30 mg/min, while monitoring the patient's vital functions and maintaining verbal contact with the patient.
If toxic symptoms appear, the injection should be stopped immediately.
Levobupivacaine Kabi
(Levobupivacaine)
5 mg/ml
Read the instructions carefully. Shake until any contents of the neck are removed.
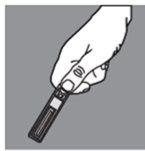
Hold the ampoule in the palm of your hand at waist level. Hold the arrow on the ampoule cap between your thumb and index finger (with your thumb outward). Twist quickly and sharply towards you (in a counter-clockwise direction).
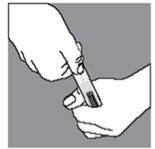
Push the Luer cone of the syringe firmly into the ampoule
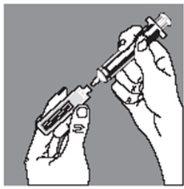
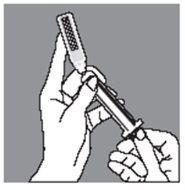
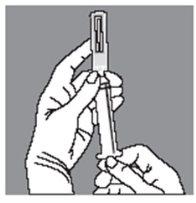
Push the ampoule gently towards you with your index finger and withdraw the contents slowly, taking special care at the beginning
- Country of registration
- Availability in pharmacies
Supply issue reported
Data from the Spanish Agency of Medicines (AEMPS) indicates a supply issue affecting this medicine.<br><br>Availability may be limited in some pharmacies.<br><br>For updates or alternatives, consult your pharmacist. - Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to LEVOBUPIVACAINE KABI 5 mg/ml INJECTABLE SOLUTION AND PERFUSION SOLUTIONDosage form: INJECTABLE PERFUSION, 0.625 mg/mLActive substance: levobupivacaineManufacturer: Altan Pharmaceuticals SaPrescription requiredDosage form: INJECTABLE PERFUSION, 1.25 mg/mlActive substance: levobupivacaineManufacturer: Altan Pharmaceuticals SaPrescription requiredDosage form: INJECTABLE, 5 mg/mlActive substance: levobupivacaineManufacturer: Altan Pharmaceuticals SaPrescription required
Online doctors for LEVOBUPIVACAINE KABI 5 mg/ml INJECTABLE SOLUTION AND PERFUSION SOLUTION
Discuss questions about LEVOBUPIVACAINE KABI 5 mg/ml INJECTABLE SOLUTION AND PERFUSION SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















