
LANSOPRAZOL SUN 30 mg ORALLY DISINTEGRATING TABLETS

How to use LANSOPRAZOL SUN 30 mg ORALLY DISINTEGRATING TABLETS
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Lansoprazole Sun 15 mg Orally Disintegrating Tablets EFG
Lansoprazole Sun 30 mg Orally Disintegrating Tablets EFG
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Lansoprazole Sun and what is it used for
- What you need to know before you take Lansoprazole Sun
- How to take Lansoprazole Sun
- Possible side effects
- Storage of Lansoprazole Sun
- Contents of the pack and other information
1. What is Lansoprazole Sun and what is it used for
The active substance of Lansoprazole Sun is lansoprazole, a proton pump inhibitor. Proton pump inhibitors reduce the amount of acid produced by the stomach.
Your doctor may prescribe this medicine for the following indications:
- Treatment of duodenal and gastric ulcers.
- Treatment of inflammation of the esophagus (reflux esophagitis)
- Prevention of reflux esophagitis
- Treatment of heartburn and acid regurgitation
- Treatment of infections caused by the bacteria Helicobacter pylori when given in combination with antibiotic therapy
- Treatment or prevention of duodenal or gastric ulcers in patients who require continuous treatment with non-steroidal anti-inflammatory drugs (NSAIDs) (NSAID treatment is used for pain or inflammation)
- Treatment of Zollinger-Ellison syndrome
2. What you need to know before you take Lansoprazole Sun
Do not take Lansoprazole Sun
- if you are allergic to lansoprazole or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Consult your doctor if you have severe liver disease. Your doctor may need to adjust your dose.
Your doctor may have performed or may perform a complementary test called endoscopy to diagnose your disease and/or rule out a malignant disease.
If you experience severe or persistent diarrhea during treatment with lansoprazole, contact your doctor immediately, as lansoprazole has been associated with a small increase in infectious diarrhea.
If your doctor has prescribed lansoprazole in addition to other medications intended for the treatment of Helicobacter pylori infection (antibiotics) or together with anti-inflammatory medications to treat pain or rheumatic disease: also read the package leaflets of these medications carefully.
If you take lansoprazole for more than three months, it is possible that your blood magnesium levels may decrease. Low magnesium levels can manifest as fatigue, involuntary muscle contractions, disorientation, convulsions, dizziness, and increased heart rate. If you experience any of these symptoms, inform your doctor immediately. Low magnesium levels can also cause a reduction in blood potassium or calcium levels. Your doctor may decide to perform periodic blood tests to monitor your magnesium levels.
Taking a proton pump inhibitor like lansoprazole, especially for a period of more than one year, may slightly increase the risk of hip, wrist, or spine fractures. Inform your doctor if you have osteoporosis (reduced bone density) or if your doctor has told you that you are at risk of osteoporosis (for example, if you are taking steroids).
This medicine may affect the way your body absorbs vitamin B12, especially if you need to take it for a prolonged period. Contact your doctor if you notice any of the following symptoms, which could indicate low vitamin B12 levels:
- Extreme fatigue or lack of energy
- Pins and needles
- Sore or red tongue, mouth ulcers
- Muscle weakness
- Altered vision
- Memory problems, confusion, depression
If you take lansoprazole for a prolonged period (more than 1 year), your doctor will likely keep you under regular control. You must report any new or exceptional symptoms and circumstances each time you consult your doctor.
Consult your doctor before taking lansoprazole:
- if you are going to have a specific blood test (Chromogranin A).
- if you have ever had a skin reaction after treatment with a medicine similar to lansoprazole that reduces stomach acid.
Severe skin reactions, including Stevens-Johnson syndrome, toxic epidermal necrolysis, and drug reaction with eosinophilia and systemic symptoms (DRESS), have been reported in association with lansoprazole treatment. Stop using lansoprazole and seek immediate medical attention if you notice any of the symptoms described in section 4.
If you have a skin rash, especially in sun-exposed areas, inform your doctor as soon as possible, as you may need to interrupt your treatment with lansoprazole. Also, mention any other adverse effect such as joint pain.
When taking lansoprazole, kidney inflammation may occur. The signs and symptoms may include decreased urine output or blood in the urine and/or hypersensitivity reactions such as fever, rash, and joint stiffness. You should report such signs to your treating doctor.
Other medicines and Lansoprazole Sun
Tell your doctor or pharmacist if you are taking, have recently taken, or might take any other medicines.
In particular, tell your doctor or pharmacist if you are using medicines that contain any of the following active substances, as lansoprazole may affect their mode of action:
- HIV protease inhibitors such as atazanavir and nelfinavir (used to treat HIV)
- methotrexate (used to treat autoimmune diseases and cancer)
- ketoconazole, itraconazole, rifampicin (used to treat infections)
- digoxin (used to treat heart problems)
- warfarin (used to treat blood clots)
- theophylline (used to treat asthma)
- tacrolimus (used to prevent transplant rejection)
- fluvoxamine (used to treat depression and other psychiatric disorders)
- antacids (used to treat stomach acidity or acid regurgitation)
- sucralfate (used to heal ulcers)
- St. John's Wort (Hypericum perforatum) (used to treat mild depression)
Taking Lansoprazole Sun with food and drinks
To get the best results from your medicine, you should take lansoprazole at least 30 minutes before meals.
Pregnancy, breastfeeding, and fertility
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine.
Driving and using machines
Sometimes side effects such as dizziness, vertigo, fatigue, and visual disturbances occur in patients taking lansoprazole. If you experience side effects like these, you should be careful as your reaction ability may be reduced. You are responsible for deciding whether you are in a fit state to drive a motor vehicle or perform other tasks that require increased attention. Due to its effects or side effects, one of the factors that can reduce your ability to do these things safely is the use of medicines.
The descriptions of these effects can be found in other sections.
Read all the information in this leaflet as guidance.
Talk to your doctor or pharmacist if you are not sure about anything.
Lansoprazole Sun contains aspartame
Each 15 mg Lansoprazole Sun tablet contains 4.5 mg of aspartame.
Each 30 mg Lansoprazole Sun tablet contains 9.0 mg of aspartame.
Aspartame is a source of phenylalanine that may be harmful in case of phenylketonuria (PKU), a rare genetic disease in which phenylalanine accumulates because the body is unable to eliminate it properly.
3. How to take Lansoprazole Sun
Follow the instructions for administration of this medicine exactly as indicated by your doctor. If in doubt, consult your doctor or pharmacist again.
Place the tablet on your tongue and suck it slowly. The tablet dissolves quickly in the mouth, releasing microgranules that should be swallowed without chewing. You can also swallow the tablet whole with a glass of water.
If you are taking lansoprazole once a day, try to take it at the same time every day. You may get better results if you take lansoprazole in the morning.
If you are taking lansoprazole twice a day, you should receive the first dose in the morning and the second dose in the evening.
The dose of lansoprazole depends on your disease. The recommended doses of lansoprazole for adults are indicated below. Your doctor will prescribe a different dose and indicate how long the treatment will last.
Treatment of heartburn and acid regurgitation:one 15 mg or 30 mg orally disintegrating tablet per day for 4 weeks. If your symptoms do not improve in 4 weeks, consult your doctor.
Treatment of duodenal ulcer:one 30 mg orally disintegrating tablet per day for 2 weeks.
Treatment of gastric ulcer:one 30 mg orally disintegrating tablet per day for 4 weeks.
Treatment of esophagus inflammation (reflux esophagitis):one 30 mg orally disintegrating tablet per day for 4 weeks.
Long-term prevention of reflux esophagitis:one 15 mg orally disintegrating tablet per day, your doctor may adjust your dose to one 30 mg orally disintegrating tablet per day.
Treatment of Helicobacter pylori infection:the usual dose is one 30 mg orally disintegrating tablet in combination with two different antibiotics in the morning and one 30 mg orally disintegrating tablet in combination with two different antibiotics in the evening. The treatment will usually be for 7 days.
The recommended combinations of antibiotics are as follows:
- 30 mg lansoprazole with 250-500 mg clarithromycin and 1000 mg amoxicillin.
- 30 mg lansoprazole with 250 mg clarithromycin and 400-500 mg metronidazole.
If you are receiving treatment for an infection because you have an ulcer, it is unlikely that the ulcer will return if the infection is successfully treated. For your medicine to have the greatest chance of working, take it at the right time and do not miss any dose.
Treatment of duodenal or gastric ulcers in patients who require continuous treatment with NSAIDs:one 30 mg orally disintegrating tablet per day for 4 weeks.
Prevention of duodenal or gastric ulcers in patients who require continuous treatment with NSAIDs:one 15 mg orally disintegrating tablet per day, your doctor may adjust your dose to one 30 mg orally disintegrating tablet per day.
Zollinger-Ellison syndrome:The usual dose is two 30 mg orally disintegrating tablets per day to start, then, depending on how you respond to lansoprazole, the dose that your doctor decides is best for you.
Use in children
Lansoprazole should not be administered to children.
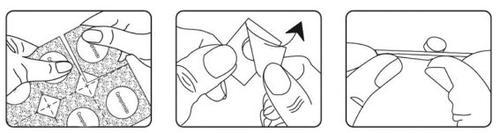
How to take
1- Hold the blister strip by the edges and separate one blister cell from the rest of the strip by gently tearing along the perforations that surround it.
2- Carefully remove the packaging.
3- Gently push the tablet out.
4- Place the tablet in your mouth. It will dissolve directly in your mouth, so you can swallow it easily.
In case of difficulty swallowing
Your doctor may indicate that you should take the tablet dissolved in a syringe, in case you have severe difficulty swallowing.
The following instructions should be followed if administered with a syringe:
It is important to carefully check the suitability of the selected syringe.
- Remove the plunger from the syringe (syringe of at least 5 ml for the 15 mg tablet and syringe of 10 ml for the 30 mg tablet)
- Insert the tablet into the syringe body.
- Replace the plunger in the syringe.
- For the 15 mg tablet: draw up 4 ml of tap water with the syringe.
- For the 30 mg tablet: draw up 10 ml of tap water with the syringe.
- Invert the syringe and draw up an additional 1 ml of air.
- Gently shake the syringe for 10-20 seconds until the tablet is dispersed.
- The contents can be poured directly into the mouth.
- Refill the syringe with 2-5 ml of tap water to remove any remaining contents and take them to the mouth.
If you use a nasogastric tube:
It is important to carefully check the suitability of the selected tube.
The recommended diameter of the nasogastric tube to be used is 3.3 mm (size 10 French) or larger.
- Remove the plunger from the syringe (use at least a 25 ml syringe for the 15 mg tablet and a 50 ml syringe for the 30 mg tablet).
- Insert the tablet into the syringe body.
- Replace the plunger in the syringe.
- For the 15 mg tablet: draw up 10 ml of tap water with the syringe.
- For the 30 mg tablet: draw up 25 ml of tap water with the syringe.
- Invert the syringe and draw up an additional 5 ml of air.
- Gently shake the syringe for 10-20 seconds until the tablet is dispersed.
- Connect the syringe to the nasogastric tube and empty the syringe contents into the tube.
- For the 15 mg tablet: refill the syringe with 10 ml of tap water and empty the syringe contents into the tube.
- For the 30 mg tablet: refill the syringe with 25 ml of tap water and empty the syringe contents into the tube.
If you take more Lansoprazole Sun than you should
If you take more lansoprazole than you have been told, seek medical attention immediately.
In case of overdose or accidental ingestion, consult your doctor or pharmacist immediately or call the Toxicology Information Service, phone 91 562 04 20, indicating the medicine and the amount ingested.
If you forget to take Lansoprazole Sun
If you forget to take a dose, take it as soon as you remember, unless it is almost time for your next dose. If this happens, omit the missed dose and take the rest of the orally disintegrating tablets as usual. Do not take a double dose to make up for missed doses.
If you stop taking Lansoprazole Sun
Do not stop treatment prematurely because your symptoms have improved. It is possible that your condition has not been completely cured and may return if you do not complete the treatment.
If you have any other questions about the use of this medicine, ask your doctor or pharmacist.
4. Possible Adverse Effects
Like all medicines, this medicine can cause adverse effects, although not all people suffer from them.
If you think you may have any of the following serious adverse effects, stop taking this medicine and contact your doctor or go immediately to the emergency room of the nearest hospital.
- Very rarely, lansoprazol can cause severe hypersensitivity (allergic) reactions. The symptoms of a hypersensitivity reaction may include fever, rash, swelling of the face, inflammation of the lymph nodes, swelling of the tongue or pharynx, difficulty swallowing, urticaria, difficulty breathing, and sometimes a drop in blood pressure.
- Very rarely, severe skin reactions have been reported with lansoprazol, which can be life-threatening. The symptoms include reddish spots on the trunk, target-shaped or circular spots, often with central blisters, skin peeling, mouth ulcers, throat, nose, genitals, and eyes. These severe skin eruptions can be preceded by fever and flu-like symptoms [Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN)].
- Generalized rash, elevated body temperature, and enlarged lymph nodes [Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS, frequency not known)].
- If you take lansoprazol for more than three months, it is possible that your blood magnesium levels may decrease. Low magnesium levels can manifest as fatigue, involuntary muscle contractions, disorientation, convulsions, dizziness, and increased heart rate. If you experience any of these symptoms, inform your doctor immediately. Low magnesium levels can also cause a reduction in blood potassium or calcium levels. Your doctor may decide to perform periodic blood tests to monitor your magnesium levels.
- Very rarely, lansoprazol can cause a reduction in the number of white blood cells (agranulocytosis) and your resistance to infections may be decreased or coexist with abnormal reductions in the number of red and white blood cells, as well as platelets (pancytopenia). If you experience an infection with symptoms such as fever and severe deterioration of your general condition, or fever with symptoms of local infection such as sore throat/pharynx/mouth or urinary problems, or you experience fatigue, pale skin with bruising or unexplained bleeding more than usual, you should consult your doctor immediately. A blood test will be performed to check for a possible reduction in blood cells.
- Rarely, lansoprazol can cause pancreatitis and its symptoms include severe and sudden pain in the middle of the upper abdomen that can radiate to the back, which can cause nausea and vomiting.
- If you experience severe or persistent diarrhea during treatment with lansoprazol, contact your doctor immediately, as lansoprazol has been associated with a small increase in infectious diarrhea.
Other Possible Adverse Effects:
Frequent:may affect up to 1 in 10 people:
- headache, dizziness
- constipation, stomach pain, discomfort, gas, dry or sore mouth/throat
- skin rash, itching
- changes in liver function test values
- fatigue
- benign stomach polyps
Uncommon:may affect up to 1 in 100 people:
- depression
- joint or muscle pain
- fluid retention or swelling
- changes in blood cell count
- risk of hip, wrist, and spine fractures
Rare:may affect up to 1 in 1,000 people:
- fever
- restlessness, drowsiness, confusion, hallucinations, insomnia, visual disturbances, vertigo
- change in taste, loss of appetite, tongue inflammation (glossitis)
- skin reactions such as burning or stinging sensation under the skin, bruising, redness, and excessive sweating
- photosensitivity
- hair loss
- tingling sensation (paresthesia), tremors
- anemia (pallor)
- kidney inflammation (interstitial nephritis), possible symptoms include changes in urine output, blood in the urine
- liver inflammation (may manifest as yellow skin or eyes)
- breast swelling in men, impotence
- candidiasis (fungal infection, may affect the esophagus mucosa)
Very Rare:may affect up to 1 in 10,000 people:
- mouth inflammation (stomatitis)
- intestinal inflammation (colitis)
- increased test values, such as cholesterol and triglyceride levels
- low sodium levels in the blood, symptoms include nausea and vomiting, headache, drowsiness, and fatigue, confusion, weakness, or muscle spasms, irritability, convulsions, coma. Your doctor may decide to perform periodic blood tests to monitor your sodium levels.
Frequency Not Known:cannot be estimated from the available data.
- lupus-like skin reactions or lupus rash
- rash, possibly with joint pain
- visual hallucinations
Reporting Adverse Effects
If you experience any type of adverse effect, consult your doctor or pharmacist, even if it is a possible adverse effect that is not listed in this prospectus. You can also report them directly through the Spanish Pharmacovigilance System for Human Use Medicines: https://www.notificaram.es. By reporting adverse effects, you can contribute to providing more information on the safety of this medicine.
5. Storage of Lansoprazol Sun
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiration date that appears on the blister pack and on the box after "CAD". The expiration date is the last day of the month indicated.
Do not store above 30°C.
Medicines should not be disposed of through wastewater or household waste. Deposit the packaging and medicines you no longer need in the SIGRE collection point at the pharmacy. In case of doubt, ask your pharmacist how to dispose of the packaging and medicines you no longer need. This will help protect the environment.
6. Package Contents and Additional Information
Lansoprazol Sun Composition
- The active ingredient is lansoprazol.
Each orodispersible tablet contains 15 mg of lansoprazol.
Each orodispersible tablet contains 30 mg of lansoprazol.
- The other components (excipients) are microcrystalline cellulose pellets, magnesium carbonate, low-substituted hydroxypropylcellulose, hydroxypropylcellulose, talc, mannitol, cornstarch, methacrylic acid and ethyl acrylate copolymer, macrogol, glycerol monostearate, polysorbate 80, citric acid, triethyl citrate, hypromellose, yellow iron oxide (E172), red iron oxide (E172), crospovidone, aspartame (E951), strawberry flavor powder, anhydrous colloidal silica, and magnesium stearate.
Product Appearance and Package Contents
Lansoprazol Sun 15 mg are white to off-white round tablets, 9 mm in diameter, with orange to dark brown spots and engraved with '15' on one side and smooth on the other.
Lansoprazol Sun 30 mg are white to off-white round tablets, 12 mm in diameter, with orange to dark brown spots and engraved with '30' on one side and smooth on the other.
Lansoprazol Sun 15 mg orodispersible tablets are packaged in precut unit-dose blisters (AL/HDPE/PE with desiccant (attached to the foil) calcium oxide in OPA/AL/HDPE/PE film), in a pack size containing 14x1, 28x1, 56x1, 98x1 orodispersible tablets.
Lansoprazol Sun 30 mg orodispersible tablets are packaged in precut unit-dose blisters (AL/HDPE/PE with desiccant (attached to the foil) calcium oxide in OPA/AL/HDPE/PE film), in a pack size containing 14x1, 28x1, 56x1, 98x1 orodispersible tablets.
Not all pack sizes may be marketed.
Marketing Authorization Holder and Manufacturer
Marketing Authorization Holder
Sun Pharmaceutical Industries Europe B.V.
Polarisavenue 87
2132JH Hoofddorp
Netherlands
Manufacturer
Sun Pharmaceutical Industries Europe B.V.
Polarisavenue 87
2132JH Hoofddorp
Netherlands
Or
Terapia S.A.
124 Fabricii Street
400 632 Cluj Napoca,
Romania
You can request more information about this medicine by contacting the local representative of the marketing authorization holder:
Sun Pharma Laboratorios, S.L.
Rambla de Catalunya 53-55
08007 Barcelona
Spain
This medicine is authorized in the Member States of the European Economic Area under the following names:
Spain: Lansoprazol SUN 15 mg orodispersible tablets EFG
Lansoprazol SUN 30 mg orodispersible tablets EFG
France: Lansoprazol SUN 15 mg orodispersible tablet
Lansoprazol SUN 30 mg orodispersible tablet
Italy: Lansoprazolo SUN Pharma
Sweden: Lansoprazole SUN pharma 15 mg orodispersible tablet
Lansoprazole SUN pharma 30 mg orodispersible tablet
Date of the Last Revision of this Prospectus:September 2024
Detailed information about this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to LANSOPRAZOL SUN 30 mg ORALLY DISINTEGRATING TABLETSDosage form: ORALLY DISINTEGRATING TABLET/LIOTAB, 15 mgActive substance: lansoprazoleManufacturer: Laboratorios Salvat S.A.Prescription requiredDosage form: ORALLY DISINTEGRATING TABLET/LIOTAB, 30 mgActive substance: lansoprazoleManufacturer: Laboratorios Salvat S.A.Prescription requiredDosage form: CAPSULE, 15 mg lansoprazoleActive substance: lansoprazoleManufacturer: Merck S.L.Prescription required
Online doctors for LANSOPRAZOL SUN 30 mg ORALLY DISINTEGRATING TABLETS
Discuss questions about LANSOPRAZOL SUN 30 mg ORALLY DISINTEGRATING TABLETS, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















