LAMPIT 30 mg TABLETS

How to use LAMPIT 30 mg TABLETS
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Lampit30 mgtablets
nifurtimox
This medicinal product is subject to additional monitoring, which will allow for the quick identification of new safety information. You can help by reporting any side effects you may experience. The last section of section 4 will tell you how to report side effects.
Read all of this leaflet carefully before you or your child start taking this medicine, because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you or your child only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you or your child experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Lampit and what is it used for
- What you need to know before you start taking Lampit
- How to take Lampit
- Possible side effects
- Storage of Lampit
- Contents of the pack and other information
1. What is Lampit and what is it used for
Lampit contains the active substance nifurtimox, which belongs to a class of medicines called nitrofuran derivatives.
Lampit is used to treat Chagas disease (American trypanosomiasis) caused by the parasite Trypanosoma cruziin adults, children, and adolescents.
2. What you need to know before you start taking Lampit
Do not take Lampit
- if you are allergic to nifurtimox or any of the other ingredients of this medicine (listed in section 6).
- if you plan to drink alcohol during treatment, as alcohol may worsen the side effects caused by Lampit. Therefore, do not drink alcohol while taking Lampit.
Warnings and precautions
Talk to your doctor or pharmacist before starting to take Lampit if:
- you have a history of brain damage, epileptic seizures (convulsions), mental health problems, or behavioral changes.
- you have a genetic disorder called porphyria (a group of disorders resulting from the accumulation of normal body chemicals called porphyrins). Taking Lampit may worsen your porphyria symptoms, including dark urine, severe stomach pain, muscle pain, tingling, numbness, mental changes, or skin sensitivity to light.
- you have severe kidney problems or are on dialysis.
- you have liver problems.
- you are pregnant or could become pregnant. Lampit may harm the fetus. A pregnancy test is recommended before starting to take Lampit. If you could become pregnant, you must use an effective contraceptive method during treatment and up to 6 months after treatment (see section 2 "Pregnancy and breastfeeding").
- you are breastfeeding. Lampit is excreted in human breast milk (see section 2 "Pregnancy and breastfeeding").
- you are a man and have a partner who could become pregnant. You must use condoms during treatment and for 3 months after treatment.
Talk to your doctor or pharmacist during treatment with Lampit if:
- you experience allergic reactions. If you experience symptoms such as low blood pressure, rapid swelling of the skin or other tissues (including swelling of the face, tongue, and/or throat), difficulty breathing, intense itching, rash, or other severe skin reactions, stop taking Lampit and contact your doctor immediately.
- you develop brain and nervous system disorders such as epileptic seizures (convulsions) or mental disorders (thought or behavioral changes). Inform your doctor or pharmacist immediately if you experience any of these symptoms during treatment.
- you lose weight or experience a loss of appetite. During treatment with Lampit, it is necessary to monitor your weight every 14 days. If you lose weight, your doctor will decide whether it is necessary to adjust the dose (see section 3 "How to take Lampit").
- you are pregnant or think you may be pregnant. Lampit may harm the fetus. Do not use Lampit during pregnancy unless your doctor has carefully considered the benefits for you and the risks for the fetus (see section 2 "Pregnancy and breastfeeding").
Children and adolescents
Lampit can be used in all age groups, from newborns to adolescents (see section 1 "What is Lampit and what is it used for").
It is not known if Lampit is safe and effective in premature newborns and in children who weigh less than 2.5 kg.
Other medicines and Lampit
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicines.
Taking Lampit with alcohol
Do not drink alcohol during treatment with Lampit (see section 2 "Do not take Lampit").
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or plan to have a baby, ask your doctor or pharmacist for advice before taking this medicine.
Pregnancy and contraception in women
Lampit may harm the fetus. Do not use Lampit during pregnancy unless your doctor has carefully considered the benefits for you and the risks for the fetus.
A pregnancy test is recommended before starting to take Lampit. If you could become pregnant, you must use an effective contraceptive method during treatment and up to 6 months after treatment.
Contraception in men
Male patients with partners who could become pregnant must use condoms during treatment and for 3 months after treatment.
Breastfeeding
Lampit is excreted in human breast milk.
Talk to your doctor if you are breastfeeding. Your doctor will carefully consider the benefits and risks of breastfeeding and taking Lampit.
Driving and using machines
Lampit may cause muscle weakness, tremors, dizziness, drowsiness, loss of balance, or agitation (see section 4 "Possible side effects"). If this happens, do not drive, do not ride a bicycle, or use any tools or machines.
Lampit contains sodium
This medicine contains less than 1 mmol of sodium (23 mg) per maximum daily dose; it is essentially "sodium-free".
3. How to take Lampit
Follow exactly the administration instructions of this medicine given by your doctor. In case of doubt, consult your doctor or pharmacist again.
The recommended dose depends on your body weight and age. Your doctor will advise you on your individualized dose and treatment plan. Your doctor may adjust your dose if your body weight changes (see section 2 "Warnings and precautions").
Lampit must be taken 3 times a day with food.
Duration of treatment
The recommended duration of treatment for adult patients (18 years or older) is 60 to 120 days.
The recommended duration of treatment for children and adolescents (under 18 years) is 60 days.
To avoid the recurrence of infection, it is important that you or your child complete the entire treatment.
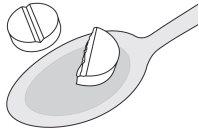
Instructions for dividing the tablets
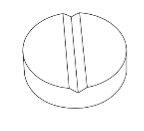
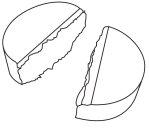
The tablets have a score line. The tablet can be divided into two equal doses.
Do not mechanically break the tablets using a tablet divider device.
The score line is used to divide the tablet by hand as follows:
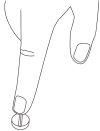
- To divide the tablet, place it on a flat surface with the score line facing up (Step 1).
- With the tablet supported on the flat surface, apply sufficient downward pressure with your index finger centered on the top of the tablet to break it along the score line (Step 2).
Step 1 | Step 2 | Two equal doses |
|
|
|
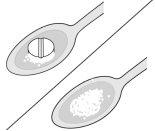
Preparation of a suspension
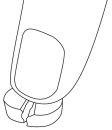
- If you or your child have difficulty swallowing the tablets, a suspension can be prepared from whole or half tablets.
- The tablet must be mixed with water (Step 1), approximately one teaspoon (5 ml), until a suspension is formed (usually takes less than 30 seconds; Step 2). The suspension must be taken immediately with food.
Step 1 | Step 2 | Suspension |
|
|
|
If you take more Lampit than you should
If you or your child have taken more Lampit than the usual dose, contact your doctor immediately.
In case of overdose, consult your doctor or pharmacist immediately or call the Toxicology Information Service, phone: 91 562 04 20, indicating the medicine and the amount taken.
If you forget to take Lampit
If you forget to take a dose, take it as soon as possible with food.
If there are less than 3 hours until the next dose, skip the missed dose and continue treatment as prescribed with the next scheduled dose.
Do not take a double dose to make up for the missed dose.
If you stop taking Lampit
Do not stop taking this medicine without consulting your doctor first. It is important that you take Lampit for the entire time your doctor has prescribed it (see section 3 "Duration of treatment"). If you cannot take the medicine as your doctor has prescribed it, contact your doctor immediately, as your illness may worsen.
If you have any other questions about the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
The following side effects may appear in adults, children, and adolescents during treatment with Lampit. However, the frequency of these side effects is unknown (the frequency cannot be estimated from the available data):
- Allergic reactions (hypersensitivity reactions)
- Feeling of spinning (vertigo)
- Abdominal pain
- Feeling of nausea (nausea)
- Vomiting
- Diarrhea
- Indigestion (dyspepsia)
- Rapid swelling under the skin in areas such as the face, tongue, throat, arms, and legs that can be life-threatening if the swelling of the throat blocks the airways (angioedema)
- Rash
- Intense itching
- Skin inflammation (dermatitis)
- Rash with itching (urticaria)
- Abnormal weight loss
- Decreased appetite
- Muscle weakness
- Joint pain
- Muscle pain
- Memory loss
- Nerve damage that causes pain or numbness, burning, tingling, and/or muscle weakness in different parts of the body (peripheral neuropathies, including polyneuropathy)
- Abnormal sensation in the skin such as burning, pinching, itching, or tingling (paresthesia)
- Tremors
- Headache
- Dizziness
- Epileptic seizures (convulsions)
- Drowsiness
- Lack of interest in activities, motivation, or energy (apathy)
- Feeling of tension or inner restlessness (agitation)
- Anxiety
- Tendency to get angry and react to small provocations and disagreements (irritability)
- Nervousness
- Confusion (disorientation)
- Changes in a person's thoughts and perceptions that make it difficult for them to recognize what is real and what is not (psychotic behavior)
- Mood changes (altered mood)
- Sleep disorders, including insomnia
- Lack or loss of strength and energy (asthenia)
- Excessive fatigue (fatigue)
- General malaise
- Fever
- Low white blood cell count (leucopenia)
Additional side effects in children and adolescents
The following side effects have been observed in a study with children and adolescents (under 18 years of age):
Very common(may affect more than 1 in 10 people)
- Headache
- Vomiting
- Decreased appetite
Common(may affect up to 1 in 10 people)
- Abdominal pain
- Dizziness
- Diarrhea
- Feeling of nausea (nausea)
- Rash with itching (urticaria)
- Rash
- Lack or loss of strength and energy (asthenia)
- Excessive fatigue (fatigue)
- Fever
- Weight loss
Uncommon(may affect up to 1 in 100 people)
- Tendency to get angry and react to small provocations and disagreements (irritability)
- Anxiety
- Epileptic seizures (convulsions)
- Abnormal sensation in the skin such as burning, pinching, itching, or tingling (paresthesia)
- Tremors
- Drowsiness
- Feeling of spinning, dizziness, or loss of balance (vertigo)
- Indigestion (dyspepsia)
- Intense itching
- Joint pain
- Muscle pain
- Low white blood cell count (leucopenia)
- Low levels of neutrophils, a type of white blood cell (neutropenia)
Reporting of side effects
If you experience any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly through the Spanish Medicines Monitoring System for Human Use: www.notificaRAM.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Lampit
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the carton and on the label of the bottle after EXP. The expiry date is the last day of the month shown.
After the first opening of the bottle, use this medicine within 67 days.
Keep the bottle tightly closed. Store it in the original package to protect it from moisture. Do not remove the sachet that helps keep the medicine dry (desiccant).
Medicines should not be disposed of via wastewater or household waste. Dispose of the packaging and any unused medicine in the SIGRE collection point at the pharmacy. Ask your pharmacist how to dispose of the packaging and any unused medicine. This will help protect the environment.
6. Contents of the pack and other information
Composition of Lampit
- The active substance is nifurtimox. Each tablet contains 30 mg of nifurtimox.
- The other ingredients are cornstarch, calcium hydrogen phosphate dihydrate, magnesium stearate, anhydrous colloidal silica, and sodium lauryl sulfate.
Appearance of the product and pack contents
Lampit 30 mg tablets are yellow, round, biconvex tablets with a score line on one side and marked with '30' on the other side.
Lampit is available in 90 ml bottles containing 100 tablets with a child-resistant cap and a desiccant. The desiccant is a material that absorbs moisture and is placed in a small container to protect the tablets from moisture.
Marketing authorization holder
Bayer Hispania, S.L.
Av. Baix Llobregat, 3-5
08970 Sant Joan Despí (Barcelona)
Spain
Manufacturer
Bayer AG
Kaiser-Wilhelm-Allee
51368 Leverkusen
Germany
This medicine is authorized in the Member States of the European Economic Area under the following names:
Germany, Spain: Lampit
Date of the last revision of this leaflet:February 2024.
Detailed information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) (http://www.aemps.gob.es/).
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to LAMPIT 30 mg TABLETSDosage form: TABLET, 120 mgActive substance: nifurtimoxManufacturer: Bayer Hispania S.L.Prescription requiredDosage form: INJECTABLE, 1.5 g meglumine antimonate/5 mlActive substance: meglumine antimonateManufacturer: Sanofi Aventis S.A.Prescription requiredDosage form: INJECTABLE, 300 mg pentamidine isethionateActive substance: pentamidine isethionateManufacturer: The Simple Pharma Company LimitedPrescription required
Online doctors for LAMPIT 30 mg TABLETS
Discuss questions about LAMPIT 30 mg TABLETS, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions